Aztreonam (injection): Difference between revisions
No edit summary |
No edit summary |
||
| Line 24: | Line 24: | ||
Including [[endometritis]] and [[pelvic cellulitis]] caused by [[Escherichia coli]], [[Klebsiella pneumoniae]], [[Enterobacter]] species including [[E. cloacae]], and [[Proteus mirabilis]]. | Including [[endometritis]] and [[pelvic cellulitis]] caused by [[Escherichia coli]], [[Klebsiella pneumoniae]], [[Enterobacter]] species including [[E. cloacae]], and [[Proteus mirabilis]]. | ||
AZACTAM is indicated for adjunctive therapy to surgery in the management of infections caused by susceptible organisms, including [[abscesses]], infections complicating hollow viscus perforations, [[cutaneous infections]], and infections of serous surfaces. AZACTAM is effective against most of the commonly encountered [[Gram-negative]] aerobic pathogens seen in general surgery | AZACTAM is indicated for adjunctive therapy to surgery in the management of infections caused by susceptible organisms, including [[abscesses]], infections complicating hollow viscus perforations, [[cutaneous infections]], and infections of serous surfaces. AZACTAM is effective against most of the commonly encountered [[Gram-negative]] aerobic pathogens seen in general surgery | ||
|adverseReactions=[[chest discomfort]], [[abdominal pain]], [[vomiting]], [[alkaline phosphatase]] raised, [[ALT]]/[[SGPT]] level raised, [[AST]]/[[SGOT]] level raised, serum [[creatinine]] raised, [[cough]], [[nasal congestion]], Pain in throat, [[wheezing]], [[fever]]. | |adverseReactions=[[chest discomfort]], [[abdominal pain]], [[vomiting]], [[alkaline phosphatase]] raised, [[ALT]]/[[SGPT]] level raised, [[AST]]/[[SGOT]] level raised, serum [[creatinine]] raised, [[cough]], [[nasal congestion]], Pain in throat, [[wheezing]], [[fever]]. | ||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | ||
| Line 42: | Line 42: | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Aztreonam in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Aztreonam in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Aztreonam in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Aztreonam in pediatric patients. | ||
|contraindications=This preparation is contraindicated in patients with known [[hypersensitivity]] to aztreonam or any other component in the formulation. | |||
|warnings=Both animal and human data suggest that AZACTAM (aztreonam injection) is rarely cross-reactive with other beta-lactam antibiotics and weakly immunogenic. Treatment with aztreonam can result in [[hypersensitivity]] reactions in patients with or without prior exposure. Careful inquiry should be made to determine whether the patient has any history of [[hypersensitivity]] reactions to any [[allergens]]. | |||
While cross-reactivity of aztreonam with other [[beta-lactam]] antibiotics is rare, this drug should be administered with caution to any patient with a history of [[hypersensitivity]] to [[beta-lactams]] (eg, [[penicillins]], [[cephalosporins]], and/or [[carbapenems]]). Treatment with aztreonam can result in [[hypersensitivity reactions]] in patients with or without prior exposure to aztreonam. If an allergic reaction to aztreonam occurs, discontinue the drug and institute supportive treatment as appropriate (eg, maintenance of ventilation, [[pressor amines]], [[antihistamines]], [[corticosteroids]]). Serious hypersensitivity reactions may require epinephrine and other emergency measures. | |||
[[Clostridium difficile–associated diarrhea]] ([[CDAD]]) has been reported with use of nearly all antibacterial agents, including AZACTAM, and may range in severity from mild [[diarrhea]] to [[fatal colitis]]. Treatment with antibacterial agents alters the [[normal flora]] of the colon leading to overgrowth of [[C. difficile]]. | |||
[[C. difficile]] produces toxins A and B which contribute to the development of [[CDAD]]. Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require [[colectomy]]. [[CDAD]] must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since [[CDAD]] has been reported to occur over 2 months after the administration of antibacterial agents. | |||
If [[CDAD]] is suspected or confirmed, ongoing antibiotic use not directed against [[C. difficile]] may need to be discontinued. Appropriate fluid and [[electrolyte management]], [[protein supplementation]], antibiotic treatment of [[C. difficile]], and surgical evaluation should be instituted as clinically indicated. | |||
Rare cases of [[toxic epidermal necrolysis]] have been reported in association with aztreonam in patients undergoing [[bone marrow transplant]] with multiple risk factors including [[sepsis]], [[radiation therapy]], and other concomitantly administered drugs associated with [[toxic epidermal necrolysis]]. | |||
|alcohol=Alcohol-Aztreonam interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Aztreonam interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 17:13, 19 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Aztreonam (injection) is a monobactam antibiotic that is FDA approved for the treatment of urinary tract infections (complicated and uncomplicated), lower respiratory tract infections, septicemia, skin and skin-structure infections, intra-abdominal infections and gynecologic infections. AZACTAM is indicated for the treatment of the following infections caused by susceptible Gram-negative microorganisms:
Urinary Tract Infections (complicated and uncomplicated)
Including pyelonephritis and cystitis (initial and recurrent) caused by Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Enterobacter cloacae, Klebsiella oxytoca, Citrobacter species, and Serratia marcescens.
Lower Respiratory Tract Infections
Including pneumonia and bronchitis caused by Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Haemophilus influenzae, Proteus mirabilis, Enterobacter species, and Serratia marcescens.
Septicemia
Caused by Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Proteus mirabilis, Serratia marcescens, and Enterobacter species.
Skin and Skin-Structure Infections
Including those associated with postoperative wounds, ulcers, and burns, caused by Escherichia coli, Proteus mirabilis, Serratia marcescens, Enterobacter species, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Citrobacter species.
Intra-abdominal Infections
Including peritonitis caused by Escherichia coli, Klebsiella species including K. pneumoniae, Enterobacter species including E. cloacae, Pseudomonas aeruginosa, Citrobacter species* including C. freundii, and Serratia species* including S. marcescens.
Gynecologic Infections
Including endometritis and pelvic cellulitis caused by Escherichia coli, Klebsiella pneumoniae, Enterobacter species including E. cloacae, and Proteus mirabilis.
AZACTAM is indicated for adjunctive therapy to surgery in the management of infections caused by susceptible organisms, including abscesses, infections complicating hollow viscus perforations, cutaneous infections, and infections of serous surfaces. AZACTAM is effective against most of the commonly encountered Gram-negative aerobic pathogens seen in general surgery. Common adverse reactions include chest discomfort, abdominal pain, vomiting, alkaline phosphatase raised, ALT/SGPT level raised, AST/SGOT level raised, serum creatinine raised, cough, nasal congestion, Pain in throat, wheezing, fever..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
AZACTAM, an intravenous solution in GALAXY plastic containers (PL 2040), is intended for intravenous use only. Dosage should be determined by susceptibility of the causative organisms, severity and site of infection, and the condition of the patient.
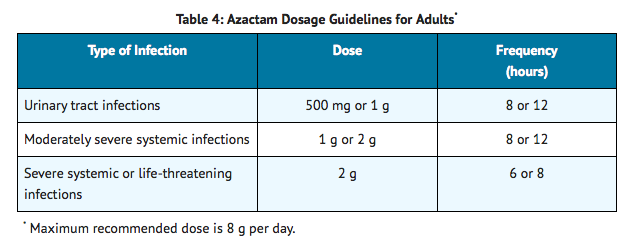
Because of the serious nature of infections due to Pseudomonas aeruginosa, dosage of 2 g every six or eight hours is recommended, at least upon initiation of therapy, in systemic infections caused by this organism.
The intravenous route is recommended for patients requiring single doses greater than 1 g or those with bacterial septicemia, localized parenchymal abscess (eg, intra-abdominal abscess), peritonitis, or other severe systemic or life-threatening infections.
The duration of therapy depends on the severity of infection. Generally, AZACTAM should be continued for at least 48 hours after the patient becomes asymptomatic or evidence of bacterial eradication has been obtained. Persistent infections may require treatment for several weeks. Doses smaller than those indicated should not be used.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aztreonam in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Aztreonam in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
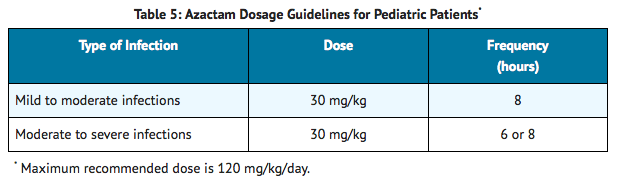
AZACTAM should be administered intravenously to pediatric patients with normal renal function. There are insufficient data regarding intramuscular administration to pediatric patients or dosing in pediatric patients with renal impairment.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aztreonam in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Aztreonam in pediatric patients.
Contraindications
This preparation is contraindicated in patients with known hypersensitivity to aztreonam or any other component in the formulation.
Warnings
Both animal and human data suggest that AZACTAM (aztreonam injection) is rarely cross-reactive with other beta-lactam antibiotics and weakly immunogenic. Treatment with aztreonam can result in hypersensitivity reactions in patients with or without prior exposure. Careful inquiry should be made to determine whether the patient has any history of hypersensitivity reactions to any allergens.
While cross-reactivity of aztreonam with other beta-lactam antibiotics is rare, this drug should be administered with caution to any patient with a history of hypersensitivity to beta-lactams (eg, penicillins, cephalosporins, and/or carbapenems). Treatment with aztreonam can result in hypersensitivity reactions in patients with or without prior exposure to aztreonam. If an allergic reaction to aztreonam occurs, discontinue the drug and institute supportive treatment as appropriate (eg, maintenance of ventilation, pressor amines, antihistamines, corticosteroids). Serious hypersensitivity reactions may require epinephrine and other emergency measures.
Clostridium difficile–associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including AZACTAM, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over 2 months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Rare cases of toxic epidermal necrolysis have been reported in association with aztreonam in patients undergoing bone marrow transplant with multiple risk factors including sepsis, radiation therapy, and other concomitantly administered drugs associated with toxic epidermal necrolysis.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Aztreonam (injection) Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Aztreonam (injection) Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Aztreonam (injection) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Aztreonam (injection) in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Aztreonam (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Aztreonam (injection) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Aztreonam (injection) in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Aztreonam (injection) in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Aztreonam (injection) in geriatric settings.
Gender
There is no FDA guidance on the use of Aztreonam (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Aztreonam (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Aztreonam (injection) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Aztreonam (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Aztreonam (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Aztreonam (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Aztreonam (injection) Administration in the drug label.
Monitoring
There is limited information regarding Aztreonam (injection) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Aztreonam (injection) and IV administrations.
Overdosage
There is limited information regarding Aztreonam (injection) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Aztreonam (injection) Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Aztreonam (injection) Mechanism of Action in the drug label.
Structure
There is limited information regarding Aztreonam (injection) Structure in the drug label.
Pharmacodynamics
There is limited information regarding Aztreonam (injection) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Aztreonam (injection) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Aztreonam (injection) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Aztreonam (injection) Clinical Studies in the drug label.
How Supplied
There is limited information regarding Aztreonam (injection) How Supplied in the drug label.
Storage
There is limited information regarding Aztreonam (injection) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Aztreonam (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Aztreonam (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Aztreonam (injection) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Aztreonam interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Aztreonam (injection) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Aztreonam (injection) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [4]
Overview
Aztreonam' (Azactam®) is a synthetic monocyclic beta-lactam antibiotic (a monobactam) originally isolated from Chromobacterium violaceum. It was approved by the FDA in 1986. It is resistant to some beta-lactamases, but is inactivated by extended-spectrum beta-lactamases.
Category
Monobactam
US Brand Names
AZACTAM®
FDA Package Insert
Description | Clinical Pharmacology | Microbiology | Indications and Usage | Contraindications | Warnings and Precautions | Adverse Reactions | Drug Interactions | Overdosage | Clinical Studies | Dosage and Administration | How Supplied | Labels and Packages
Mechanism of Action
Aztreonam is similar in action to penicillin. It inhibits mucopeptide synthesis in the bacterial cell wall. It has a very high affinity for penicillin-binding protein 3 (PBP-3) and mild affinity for PBP-1a. Aztreonam binds the penicillin-binding proteins of gram-positive and anaerobic bacteria very poorly and is largely ineffective against them.[1] Aztreonam is bactericidal but less so than some of the cephalosporins.
References
- ↑ AHFS DRUG INFORMATION® 2006 (2006 ed ed.). American Society of Health-System Pharmacists. 2006.