Atrial fibrillation pathophysiology: Difference between revisions
| Line 36: | Line 36: | ||
**When function normally these regions have a synchronous electrical activity, which can act as a trigger by producing delayed afterdepolarizations. | **When function normally these regions have a synchronous electrical activity, which can act as a trigger by producing delayed afterdepolarizations. | ||
* [[Atrial]] stretch | * [[Atrial]] stretch | ||
**It could be result of [[hypertension]], [[heart failure]] or [[valvular heart disease]] | |||
**Decreased [[resting potential]] and [[action potential]] velocity due to the stretched [[atrium]] | **Decreased [[resting potential]] and [[action potential]] velocity due to the stretched [[atrium]] | ||
* [[Bradycardia]] | * [[Bradycardia]] | ||
Revision as of 16:46, 24 August 2021
| https://https://www.youtube.com/watch?v=6FLE6HWiImM%7C350}} |
| Resident Survival Guide |
 |
Sinus rhythm  |
Atrial fibrillation 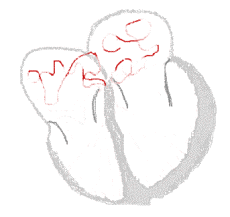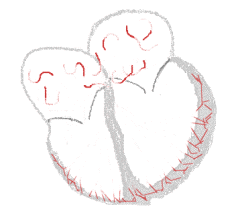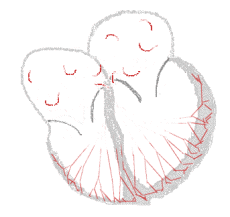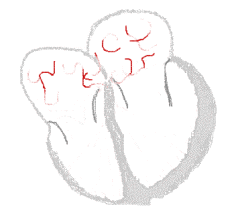 |
|
Atrial Fibrillation Microchapters | |
|
Special Groups | |
|---|---|
|
Diagnosis | |
|
Treatment | |
|
Cardioversion | |
|
Anticoagulation | |
|
Surgery | |
|
Case Studies | |
|
Atrial fibrillation pathophysiology On the Web | |
|
Directions to Hospitals Treating Atrial fibrillation pathophysiology | |
|
Risk calculators and risk factors for Atrial fibrillation pathophysiology | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Syed Hassan A. Kazmi BSc, MD [2] Anahita Deylamsalehi, M.D.[3]
Overview
The primary pathologic or structural change observed in patients with atrial fibrillation is progressive fibrosis of the atria. This fibrosis is primarily due to atrial dilatation, however genetic causes and inflammation may also play a role in some individuals. There are other functional processes that contribute to the development and persistence of atrial fibrillation including hemodynamic stress (stretching of the atrium), atrial ischemia, activation of the neurohormonal system, ectopic activity in the pulmonary vein, multiple wavelets of electrical activity in the atrium, and catecholamine excess. The mechanism in most patients is likely to be multifactorial.
Pathophysiology
Pathogenesis
Onset of atrial fibrillation is dependent upon specific triggers and tissue substrates capable of maintaining atrial fibrillation. The following triggers are know to initiate atrial fibrillation:[1][2][3][4]
- Sympathetic or parasympathetic stimulation
- Ectopic activity in muscular sleeves that extend from the left atrium into the proximal parts of pulmonary veins
- The aforementioned regions are a mixture of atrial myocardium and smooth muscle in coronary sinus and atrioventricular valves.
- When function normally these regions have a synchronous electrical activity, which can act as a trigger by producing delayed afterdepolarizations.
- Atrial stretch
- It could be result of hypertension, heart failure or valvular heart disease
- Decreased resting potential and action potential velocity due to the stretched atrium
- Bradycardia
- Premature atrial beats
- Accessory AV (atrio-ventricular) pathways
Ectopic Foci in the Pulmonary Vein
- Younger patients with paroxysmal atrial fibrillation may have ectopic foci of electrical activity in the pulmonary vein that can be ablated by the radiofrequency. [5][3][6]
- There are some cells in the pulmonary vein whose electrical properties resemble those of the myocytes of the atrium and are responsible for the rapid discharge through the atrium.[3]
- These patients generally have high grade ectopic activity on Holter monitoring.
- While the pulmonary vein is a common source of these ectopic foci, there may also be foci present in the atrium itself.
- While the pulmonary vein may function as a trigger, it is the heterogeneity of conduction that may sustain the atrial fibrillation.
- Methods such as fluoroscopy, angiographic imaging and intracardiac mapping have been detected pulmonary vein as the responsible origin of this arrythmia.
- Based on a study done in 1998 on 45 patients with atrial fibrillation demonstrated that in 94% of cases pulmonary vein is the origin of abnormal electrical activity.[3]
- Unfortunately the reason why the pulmonary vein turns to an arrhythmogenic foci is not fully understood. It seems that structure of the pulmonary vein makes it potential for re-entry formation which can lead to atrial fibrillation.[7]
Re-enterant Wavelets or Multiple Wavelets Phenomenon
- Presence of these triggers produce re-enterant wavelets of electrical activity due to shortened effective refractory period (ERP)
- It has been hypothesized that if there is a greater atrial mass, delayed atrial conduction times, and a shortened atrial refractory period, it promotes the propagation of wavelets.[8] This hypothesis is supported by the observation that prolongation of intra atrial conduction times is associated with a recurrence of atrial fibrillation.
Molecular Pathogenesis and Role of Mechano-electric Feedback
- Mechanosensitivity of cardiac myocytes is thought to play a pivotal role in initiation of atrial fibrillation:[9]
Altered myocyte stress/strain
- Alteration of extracellular matrix strain of the myocytes leads to opening of specific stretch-activated channels (SAC) via cytoskeletal linkages to integrins.[10][11]
Catecholamine Release Secondary to Atrial Stretch
- Stress/strain may lead to release of catecholamines leading to alpha and beta receptor activation in regions of greatest hemodynamic stress, as occurs in states of hypertension, mitral valvulitis and congestive heart failure.[12]
- Animal studies have shown that the gap junction protein connexin 43 (Cx43) plays a key role in electrical conduction velocity in cardiac tissues, and under expression of Cx43 is linked with AF(especially in sympathetic AF).[13][14]
Actiavtion of G-protein Coupled Pathways
- Stimulation of alpha and beta receptors leads to downstream activation of G-protein coupled pathways within the cardiac myocytes.[15][16]
- Beta adrenergic stimulation leads to activation of adenlyl cyclase and in turn increased cyclic adenosine monophosphate (cAMP) production.[17]
- Alpha receptor stimulation causes activation of phosphatidylinositol (PI) second messenger system that, via phospholipase C (PLC) action, synthesizes inositol triphosphate (IP3) and diacylglycerol (DAG).[12]
- Protein kinases A (PKA) and C (PKC), activated by cAMP and PI pathways, respectively, produce a change in intra-cellular calcium level, via opening of L-type calcium channels and sarcoplasmic reticulum (SR) calcium release.
- Moreover, protein kinase C causes mitogen-activated protein kinase (MAPK) to be activated downstream, which turns on immediate early gene (IEG) program to initiate hypertrophy and cardiac remodelling. This produces a vicious cycle that maintains an arrhythmogenic environment.[18]
- The calcium influx due to opening to L-type calcium channels is a regulator of atrial excitation-contraction coupling.[19]
Role of Dilation of the Atria/Atrial Stress
Dilatation of the atria can be due to almost any structural abnormality of the heart that can cause a rise in the intra-cardiac pressures. This includes:
- Hypertension, most likely the most common cause of atrial dilation in the current era.
- Valvular heart disease (such as mitral stenosis, mitral regurgitation, and tricuspid regurgitation).
- Congestive heart failure.
- Coronary Artery Bypass Graft Surgery.
- Once dilatation of the atria has occurred, this begins a chain of events that leads to the activation of the renin aldosterone angiotensin system (RAAS) and subsequent increase in matrix metaloproteinases and disintegrin, which leads to atrial remodeling and fibrosis, with loss of atrial muscle mass.
- Dilation and stress may lead to decreased resting potential, action potential amplitude and duration, and occurrence of afterdepolarizations causing extrasystoles.
Inflammation of the Atria
- Any inflammatory state that affects the heart can cause fibrosis of the atria. This is typically due to sarcoidosis but may also be due to autoimmune disorders that create autoantibodies against myosin heavy chains. Mutation of the lamin AC gene is also associated with fibrosis of the atria that can lead to atrial fibrillation.
Stretch-Induced Depolarization of Fibroblasts
- Patchy atrial fibrosis may precede the occurrence of atrial fibrillation and the magnitude of fibrosis may progress with a prolonged duration of atrial fibrillation.
Fibrosis of the SA Node
- Fibrosis is not limited to the muscle mass of the atria, and may occur in the sinus node (SA node) and atrioventricular node (AV node), correlating with sick sinus syndrome. Prolonged episodes of atrial fibrillation have been shown to correlate with prolongation of the sinus node recovery time,[20][21][22] suggesting that dysfunction of the SA node is progressive with prolonged episodes of atrial fibrillation.
Genetics
Associated Conditions
- The presence of atrial fibrillation often reflects the presence of an underlying cardiac or lung disease. Indeed, the proportion of patients with lone atrial fibrillation is low (approximately 12% of cases). [2][23][24][25][26][27]
- Conditions associated with atrial fibrillation include:[20][27][2]
Gross Pathology
Microscopic Patholohy
References
- ↑ Wit AL, Boyden PA (March 2007). "Triggered activity and atrial fibrillation". Heart Rhythm. 4 (3 Suppl): S17–23. doi:10.1016/j.hrthm.2006.12.021. PMC 1855225. PMID 17336878.
- ↑ 2.0 2.1 2.2 Allessie MA, Boyden PA, Camm AJ, Kléber AG, Lab MJ, Legato MJ; et al. (2001). "Pathophysiology and prevention of atrial fibrillation". Circulation. 103 (5): 769–77. doi:10.1161/01.cir.103.5.769. PMID 11156892.
- ↑ 3.0 3.1 3.2 3.3 Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G; et al. (1998). "Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins". N Engl J Med. 339 (10): 659–66. doi:10.1056/NEJM199809033391003. PMID 9725923.
- ↑ Mary-Rabine L, Albert A, Pham TD, Hordof A, Fenoglio JJ, Malm JR; et al. (1983). "The relationship of human atrial cellular electrophysiology to clinical function and ultrastructure". Circ Res. 52 (2): 188–99. doi:10.1161/01.res.52.2.188. PMID 6218936.
- ↑ Iwasaki YK, Nishida K, Kato T, Nattel S (2011). "Atrial fibrillation pathophysiology: implications for management". Circulation. 124 (20): 2264–74. doi:10.1161/CIRCULATIONAHA.111.019893. PMID 22083148.
- ↑ Nishida K, Maguy A, Sakabe M, Comtois P, Inoue H, Nattel S (2011). "The role of pulmonary veins vs. autonomic ganglia in different experimental substrates of canine atrial fibrillation". Cardiovasc Res. 89 (4): 825–33. doi:10.1093/cvr/cvq332. PMID 20962102.
- ↑ Po SS, Li Y, Tang D, Liu H, Geng N, Jackman WM; et al. (2005). "Rapid and stable re-entry within the pulmonary vein as a mechanism initiating paroxysmal atrial fibrillation". J Am Coll Cardiol. 45 (11): 1871–7. doi:10.1016/j.jacc.2005.02.070. PMID 15936621.
- ↑ Akyürek O, Sayin T, Dinçer I, Karaoguz R, Güldal M, Oral D. Lengthening of intraatrial conduction time in atrial fibrillation and its relation with early recurrence of atrial fibrillation. Jpn Heart J. Sep 2001;42(5):575-84.
- ↑ Franz, M (2000). "Mechano-electrical feedback". Cardiovascular Research. 45 (2): 263–266. doi:10.1016/S0008-6363(99)00390-9. ISSN 0008-6363.
- ↑ Sadoshima, Junichi; Izumo, Seigo (1997). "THE CELLULAR AND MOLECULAR RESPONSE OF CARDIAC MYOCYTES TO MECHANICAL STRESS". Annual Review of Physiology. 59 (1): 551–571. doi:10.1146/annurev.physiol.59.1.551. ISSN 0066-4278.
- ↑ Sackin, H (1995). "Mechanosensitive Channels". Annual Review of Physiology. 57 (1): 333–353. doi:10.1146/annurev.ph.57.030195.002001. ISSN 0066-4278.
- ↑ 12.0 12.1 "pdfs.semanticscholar.org" (PDF).
- ↑ Shu C, Huang W, Zeng Z, He Y, Luo B, Liu H, Li J, Xu J (July 2017). "Connexin 43 is involved in the sympathetic atrial fibrillation in canine and canine atrial myocytes". Anatol J Cardiol. 18 (1): 3–9. doi:10.14744/AnatolJCardiol.2017.7602. PMC 5512195. PMID 28554986.
- ↑ Kontogeorgis A, Li X, Kang EY, Feig JE, Ponzio M, Kang G, Kaba RA, Wit AL, Fisher EA, Morley GE, Peters NS, Coetzee WA, Gutstein DE (November 2008). "Decreased connexin43 expression in the mouse heart potentiates pacing-induced remodeling of repolarizing currents". Am. J. Physiol. Heart Circ. Physiol. 295 (5): H1905–16. doi:10.1152/ajpheart.590.2008. PMC 2614590. PMID 18757477.
- ↑ Satoh T, Zipes DP (September 1996). "Unequal atrial stretch in dogs increases dispersion of refractoriness conducive to developing atrial fibrillation". J. Cardiovasc. Electrophysiol. 7 (9): 833–42. PMID 8884512.
- ↑ Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM (September 1999). "Atrial L-type Ca2+ currents and human atrial fibrillation". Circ. Res. 85 (5): 428–36. PMID 10473672.
- ↑ Krapivinsky G, Gordon EA, Wickman K, Velimirović B, Krapivinsky L, Clapham DE (March 1995). "The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K(+)-channel proteins". Nature. 374 (6518): 135–41. doi:10.1038/374135a0. PMID 7877685.
- ↑ Goette A, Lendeckel U, Klein HU (May 2002). "Signal transduction systems and atrial fibrillation". Cardiovasc. Res. 54 (2): 247–58. PMID 12062330.
- ↑ Matsuda N, Hagiwara N, Shoda M, Kasanuki H, Hosoda S (April 1996). "Enhancement of the L-type Ca2+ current by mechanical stimulation in single rabbit cardiac myocytes". Circ. Res. 78 (4): 650–9. PMID 8635223.
- ↑ 20.0 20.1 Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL (2006). "ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society". Circulation. 114 (7): e257–354. doi:10.1161/CIRCULATIONAHA.106.177292. PMID 16908781. Unknown parameter
|month=ignored (help) - ↑ Elvan A, Wylie K, Zipes DP (1996). "Pacing-induced chronic atrial fibrillation impairs sinus node function in dogs. Electrophysiological remodeling". Circulation. 94 (11): 2953–60. PMID 8941126. Unknown parameter
|month=ignored (help) - ↑ Manios EG, Kanoupakis EM, Mavrakis HE, Kallergis EM, Dermitzaki DN, Vardas PE (2001). "Sinus pacemaker function after cardioversion of chronic atrial fibrillation: is sinus node remodeling related with recurrence?". Journal of Cardiovascular Electrophysiology. 12 (7): 800–6. PMID 11469431. Unknown parameter
|month=ignored (help) - ↑ Kopecky SL, Gersh BJ, McGoon MD; et al. (1987). "The natural history of lone atrial fibrillation. A population-based study over three decades". N. Engl. J. Med. 317 (11): 669–74. PMID 3627174. Unknown parameter
|month=ignored (help) - ↑ Furberg CD, Psaty BM, Manolio TA, Gardin JM, Smith VE, Rautaharju PM (1994). "Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study)". Am. J. Cardiol. 74 (3): 236–41. PMID 8037127. Unknown parameter
|month=ignored (help) - ↑ EVANS W, SWANN P (1954). "Lone auricular fibrillation". Br Heart J. 16 (2): 189–94. PMC 479515. PMID 13160271. Unknown parameter
|month=ignored (help) - ↑ Brand FN, Abbott RD, Kannel WB, Wolf PA (1985). "Characteristics and prognosis of lone atrial fibrillation. 30-year follow-up in the Framingham Study". JAMA. 254 (24): 3449–53. PMID 4068186. Unknown parameter
|month=ignored (help) - ↑ 27.0 27.1 Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S (2014). "Epidemiology of atrial fibrillation: European perspective". Clin Epidemiol. 6: 213–20. doi:10.2147/CLEP.S47385. PMC 4064952. PMID 24966695.