Alcohol: Difference between revisions
Kiran Singh (talk | contribs) No edit summary |
Kiran Singh (talk | contribs) No edit summary |
||
| Line 3: | Line 3: | ||
atom is attached to other carbon or hydrogen atoms.]] | atom is attached to other carbon or hydrogen atoms.]] | ||
{{SI}} | {{SI}} | ||
{{CMG | {{CMG}} | ||
==Overview== | ==Overview== | ||
| Line 88: | Line 88: | ||
The formation of a secondary alcohol via reduction and hydration is shown: | The formation of a secondary alcohol via reduction and hydration is shown: | ||
[[Image:Alcohol prep.jpg|center|350px|Preparation of a secondary alcohol]] | [[Image:Alcohol prep.jpg|center|350px|Preparation of a secondary alcohol]] | ||
==Reactions==<!-- This section is linked from [[Organic reaction]] --> | ==Reactions==<!-- This section is linked from [[Organic reaction]] --> | ||
===Deprotonation=== | ===Deprotonation=== | ||
Latest revision as of 13:59, 31 October 2014
|
WikiDoc Resources for Alcohol |
|
Articles |
|---|
|
Most recent articles on Alcohol |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Alcohol at Clinical Trials.gov Clinical Trials on Alcohol at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Alcohol
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating Alcohol Risk calculators and risk factors for Alcohol
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Alcohol |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
In layman's terms, the word alcohol (Arabic:الكحل al-kuḥl) usually refers to ethanol, also known as grain alcohol or (older) spirits of wine, or to any alcoholic beverage. Ethanol is a colorless, volatile liquid with a mild odor which can be obtained by the fermentation of sugars. (Industrially, it is more commonly obtained by ethylene hydration — the reaction of ethylene with water in the presence of phosphoric acid.[1]) Ethanol is the most widely used depressant in the world, and has been for thousands of years. This sense underlies the term alcoholism (addiction to alcohol).
In chemistry, an alcohol is any organic compound in which a hydroxyl group (-OH) is bound to a carbon atom of an alkyl or substituted alkyl group. The general formula for a simple acyclic alcohol is CnH2n+1OH.
Other alcohols are usually described with a clarifying adjective, as in isopropyl alcohol (propan-2-ol) or wood alcohol (methyl alcohol, or methanol). The suffix -ol appears in the "official" IUPAC chemical name of all alcohols.
There are three major, subsets of alcohols: 'primary' (1°), 'secondary' (2°) and 'tertiary' (3°), based upon the number of carbons the C-OH carbon (shown in red) is bonded to. Ethanol is a simple 'primary' alcohol. The simplest secondary alcohol is isopropyl alcohol (propan-2-ol), and a simple tertiary alcohol is tert-butyl alcohol (2-methylpropan-2-ol).
The phenols with parent compound phenol have a hydroxyl group (attached to a benzene ring) just like alcohols, but differ sufficiently in properties as to warrant a separate treatment.
Carbohydrates (sugars) and sugar alcohols are an important class of compounds containing multiple alcohol functional groups. For example, sucrose (common sugar) contains eight hydroxyl groups per molecule and sorbitol has six. Most of the attributes of these polyols, from nomenclature, to occurrence, use and toxicity, are sufficiently different from simple aliphatic alcohols as to require a separate treatment.
Simple alcohols
The simplest and most commonly used alcohols are methanol and ethanol. Methanol was formerly obtained by the distillation of wood and called "wood alcohol." It is now a cheap commodity, the chemical product of carbon monoxide reacting with hydrogen under high pressure.[citation needed] Methanol is intoxicating but not directly poisonous. It is toxic by its breakdown (toxication) by the enzyme alcohol dehydrogenase in the liver by forming formic acid and formaldehyde which cause permanent blindness by destruction of the optic nerve.[2]
Apart from its familiar role in alcoholic beverages, ethanol is also used as a highly controlled industrial solvent and raw material. To avoid the high taxes on ethanol for consumption, additives are added to make it unpalatable (such as denatonium benzoate — "Bitrex") or poisonous (such as methanol). Ethanol in this form is known generally as denatured alcohol; when methanol is used, it may be referred to as methylated spirits ("Meths") or "surgical spirits".
Two other alcohols whose uses are relatively widespread (though not so much as those of methanol and ethanol) are propanol and butanol. Like ethanol, they can be produced by fermentation processes. (However, the fermenting agent is a bacterium, Clostridium acetobutylicum, that feeds on cellulose, not sugars like the Saccharomyces yeast that produces ethanol.)
Nomenclature
Systematic names
In the IUPAC system, the name of the alkane chain loses the terminal "e" and adds "ol", e.g. "methanol" and "ethanol".[3] When necessary, the position of the hydroxyl group is indicated by a number between the alkane name and the "ol": propan-1-ol for CH3CH2CH2OH, propan-2-ol for CH3CH(OH)CH3. Sometimes, the position number is written before the IUPAC name: 1-propanol and 2-propanol. If a higher priority group is present (such as an aldehyde, ketone or carboxylic acid), then it is necessary to use the prefix "hydroxy",[3] for example: 1-hydroxy-2-propanone (CH3COCH2OH).
Some examples of simple alcohols and how to name them:
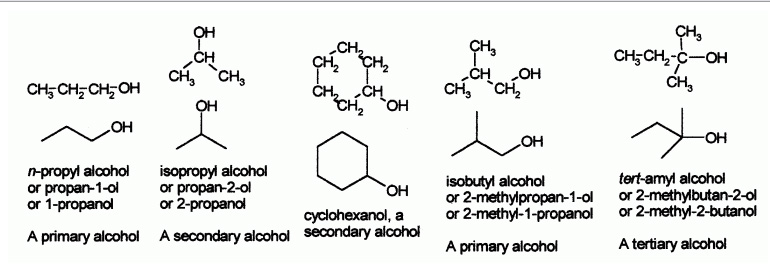
Common names for alcohols usually takes name of the corresponding alkyl group and add the word "alcohol", e.g. methyl alcohol, ethyl alcohol or tert-butyl alcohol. Propyl alcohol may be n-propyl alcohol or isopropyl alcohol depending on whether the hydroxyl group is bonded to the 1st or 2nd carbon on the propane chain. Isopropyl alcohol is also occasionally called sec-propyl alcohol.
As mentioned above alcohols are classified as primary (1°), secondary (2°) or tertiary (3°), and common names often indicate this in the alkyl group prefix. For example (CH3)3COH is a tertiary alcohol is commonly known as tert-butyl alcohol. This would be named 2-methylpropan-2-ol under IUPAC rules, indicating a propane chain with methyl and hydroxyl groups both attached to the middle (#2) carbon.
Etymology
The word "alcohol" almost certainly comes from the Arabic language (the "al-" prefix being the Arabic definite article); however, the precise origin is unclear. The Persian physician and scientist Rhazes discovered this substance, but because he wanted his book to be published in most of the then-known world, he used the Arabic language instead of Persian (although he made copies in Persian). The word was introduced into Europe, together with the art of distillation and the substance itself, around the 12th century by various European authors who translated and popularized the discoveries of Islamic and Persian alchemists.[4]
A popular theory, found in many dictionaries, is that it comes from الكحل al-kuḥl, originally the name of very finely powdered antimony sulfide Sb2S3 used as an antiseptic and eyeliner. The powder is prepared by sublimation of the natural mineral stibnite in a closed vessel. According to this theory, the meaning of alkuhul would have been first extended to distilled substances in general, and then narrowed to ethanol. This conjectured etymology has been circulating in England since 1672 at least (OED).
However, this derivation is suspicious since the current Arabic name for alcohol, الكحول Template:ISOtranslit, does not derive from Template:ISOtranslit.[citation needed] The Qur'an, in verse 37:47, uses the word الغول Template:ISOtranslit — properly meaning "spirit" or "demon" — with the sense "the thing that gives the wine its headiness". The word Template:ISOtranslit is also the origin of the English word "ghoul", and the name of the star Algol. This derivation would, of course, be consistent with the use of "spirit" or "spirit of wine" as synonymous of "alcohol" in most Western languages.
According to the second theory, the popular etymology and the spelling "alcohol" would not be due to generalization of the meaning of al-kuḥl, but rather to Western alchemists and authors confusing the two words al-kuḥl and al-ghawl, which have indeed been transliterated in many different and overlapping ways.
Physical and chemical properties
The hydroxyl group generally makes the alcohol molecule polar. Those groups can form hydrogen bonds to one another and to other compounds. This hydrogen bonding means that alcohols can be used as protic solvents. Two opposing solubility trends in alcohols are: the tendency of the polar OH to promote solubility in water, and of the carbon chain to resist it. Thus, methanol, ethanol, and propanol are miscible in water because the hydroxyl group wins out over the short carbon chain. Butanol, with a four-carbon chain, is moderately soluble because of a balance between the two trends. Alcohols of five or more carbons (Pentanol and higher) are effectively insoluble in water because of the hydrocarbon chain's dominance. All simple alcohols are miscible in organic solvents.
Because of hydrogen bonding, alcohols tend to have higher boiling points than comparable hydrocarbons and ethers. The boiling point of the alcohol ethanol is 78.29 °C, compared to 69 °C for the hydrocarbon Hexane (a common constituent of gasoline), and 34.6 °C for Diethyl ether.
Alcohols, like water, can show either acidic or basic properties at the O-H group. With a pKa of around 16-19 they are generally slightly weaker acids than water, but they are still able to react with strong bases such as sodium hydride or reactive metals such as sodium. The salts that result are called alkoxides, with the general formula RO- M+.
Meanwhile the oxygen atom has lone pairs of nonbonded electrons that render it weakly basic in the presence of strong acids such as sulfuric acid. For example, with methanol:
Alcohols can also undergo oxidation to give aldehydes, ketones or carboxylic acids, or they can be dehydrated to alkenes. They can react to form ester compounds, and they can (if activated first) undergo nucleophilic substitution reactions. The lone pairs of electrons on the oxygen of the hydroxyl group also makes alcohols nucleophiles. For more details see the reactions of alcohols section below.
Applications
Automotive
A 50 % v/v solution of ethylene glycol in water is commonly used as an antifreeze.
Some alcohols, mainly ethanol and methanol, can be used as an automotive fuel.
Performance can be increased in forced induction internal combustion engines by injecting alcohol into the air intake after the turbocharger or supercharger has pressurized the air. This cools the pressurized air, providing a denser air charge, which allows for more fuel, and therefore more power.
Scientific, medical, and industrial
Alcohols have applications in industry and science as reagents or solvents. Because of its low toxicity and ability to dissolve non-polar substances, ethanol can be used as a solvent in medical drugs, perfumes, and vegetable essences such as vanilla. In organic synthesis, alcohols serve as versatile intermediates.
Ethanol can be used as an antiseptic to disinfect the skin before injections are given, often along with iodine. Ethanol-based soaps are becoming common in restaurants and are convenient because they do not require drying due to the volatility of the compound. Alcohol is also used as a preservative for specimens.
Production
Industrially alcohols are produced in several ways:
- By fermentation using glucose produced from sugar from the hydrolysis of starch, in the presence of yeast and temperature of less than 37°C to produce ethanol. For instance the conversion of invertase to glucose and fructose or the conversion of glucose to zymase and ethanol.
- By direct hydration using ethylene (ethylene hydration[1] or other alkenes from cracking of fractions of distilled crude oil. It usually uses a catalyst of phosphoric acid under high temperature and pressure of 50-120.[citation needed]
- Methanol is produced from synthesis gas, where carbon monoxide and 2 equivalents of hydrogen gas are combined to produce methanol using a copper, zinc oxide and aluminum oxide catalyst at 250°C and a pressure of 50-100 atm.[citation needed]
Laboratory synthesis
Several methods exist for the preparation of alcohols in the laboratory.
- Primary alkyl halides react with aqueous NaOH or KOH mainly to primary alcohols in [[nucleophilic aliphatic substitution. (Secondary and especially tertiary alkyl halides will give the elimination (alkene) product instead).
- Aldehydes or ketones are reduced with sodium borohydride or lithium aluminium hydride (after an acidic workup). Another reduction by aluminumisopropylates is the Meerwein-Ponndorf-Verley reduction.
- Alkenes engage in an acid catalysed hydration reaction using concentrated sulfuric acid as a catalyst which gives usually secondary or tertiary alcohols.
- The hydroboration-oxidation and oxymercuration-reduction of alkenes are more reliable in organic synthesis.
- Grignard reagents react with carbonyl groups to secondary and tertiary alcohols
- Noyori asymmetric hydrogenation is the asymmetric reduction of β-keto-esters
The formation of a secondary alcohol via reduction and hydration is shown:

Reactions
Deprotonation
Alcohols can behave as weak acids, undergoing deprotonation. The deprotonation reaction to produce an alkoxide salt is either performed with a strong base such as sodium hydride or n-butyllithium, or with sodium or potassium metal.
- 2 R-OH + 2Na → 2R-O−Na + H2
- E.g. 2 CH3CH2-OH + 2 Na → 2 CH3-CH2-O−Na + H2
Water is similar in pKa to many alcohols, so with sodium hydroxide there is an equilibrium set up which usually lies to the left:
- R-OH + NaOH <=> R-O-Na+ + H2O (equilibrium to the left)
It should be noted, though, that the bases used to deprotonate alcohols are strong themselves. The bases used and the alkoxides created are both highly moisture sensitive chemical reagents.
The acidity of alcohols is also affected by the overall stability of the alkoxide ion. Electron-withdrawing groups attached to the carbon containing the hydroxyl group will serve to stabilize the alkoxide when formed, thus resulting in greater acidity. On the other hand, the presence of electron-donating group will result in a less stable alkoxide ion formed. This will result in a scenario whereby the unstable alkoxide ion formed will tend to accept a proton to reform the original alcohol.
With alkyl halides alkoxides give rise to ethers in the Williamson ether synthesis.
Nucleophilic substitution
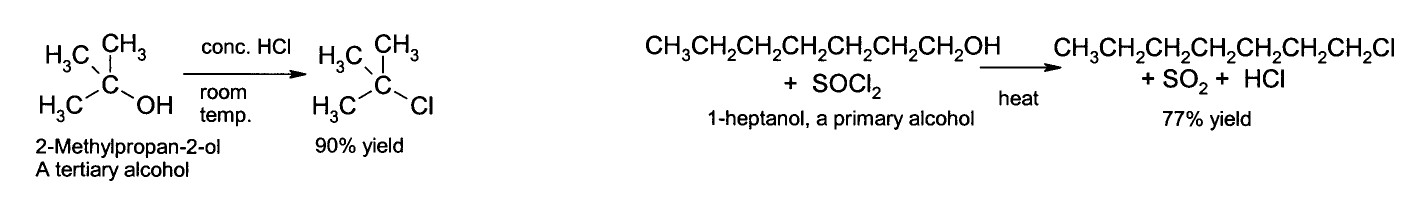
The OH group is not a good leaving group in nucleophilic substitution reactions, so neutral alcohols do not react in such reactions. However if the oxygen is first protonated to give R−OH2+, the leaving group (water) is much more stable, and nucleophilic substitution can take place. For instance, tertiary alcohols react with hydrochloric acid to produce tertiary alkyl halides, where the hydroxyl group is replaced by a chlorine atom. If primary or secondary alcohols are to be reacted with hydrochloric acid, an activator such as zinc chloride is needed. Alternatively the conversion may be performed directly using thionyl chloride.[1]
Alcohols may likewise be converted to alkyl bromides using hydrobromic acid or phosphorus tribromide, for example:
- 3 R-OH + PBr3 → 3 RBr + H3PO3
In the Barton-McCombie deoxygenation an alcohol is deoxygenated to an alkane with tributyltin hydride or a trimethylborane-water complex in a radical substitution reaction.
Dehydration
Alcohols are themselves nucleophilic, so R−OH2+ can react with ROH to produce ethers and water in a dehydration reaction, although this reaction is rarely used except in the manufacture of diethyl ether.
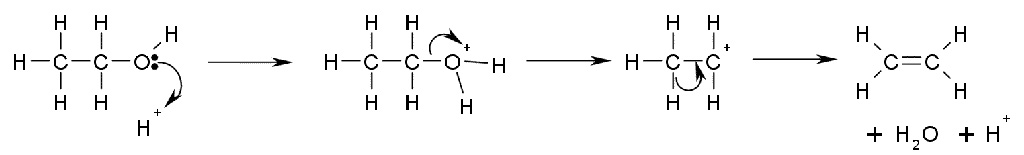
More useful is the E1 elimination reaction of alcohols to produce alkenes. The reaction generally obeys Zaitsev's Rule, which states that the most stable (usually the most substituted) alkene is formed. Tertiary alcohols eliminate easily at just above room temperature, but primary alcohols require a higher temperature.
This is a diagram of acid catalysed dehydration of ethanol to produce ethene:
A more controlled elimination reaction is the Chugaev elimination with carbon disulfide and iodomethane.
Esterification
To form an ester from an alcohol and a carboxylic acid the reaction, known as Fischer esterification, is usually performed at reflux with a catalyst of concentrated sulfuric acid:
- R-OH + R'-COOH → R'-COOR + H2O
In order to drive the equilibrium to the right and produce a good yield of ester, water is usually removed, either by an excess of H2SO4 or by using a Dean-Stark apparatus. Esters may also be prepared by reaction of the alcohol with an acid chloride in the presence of a base such as pyridine.
Other types of ester are prepared similarly- for example tosyl (tosylate) esters are made by reaction of the alcohol with p-toluenesulfonyl chloride in pyridine.
Oxidation
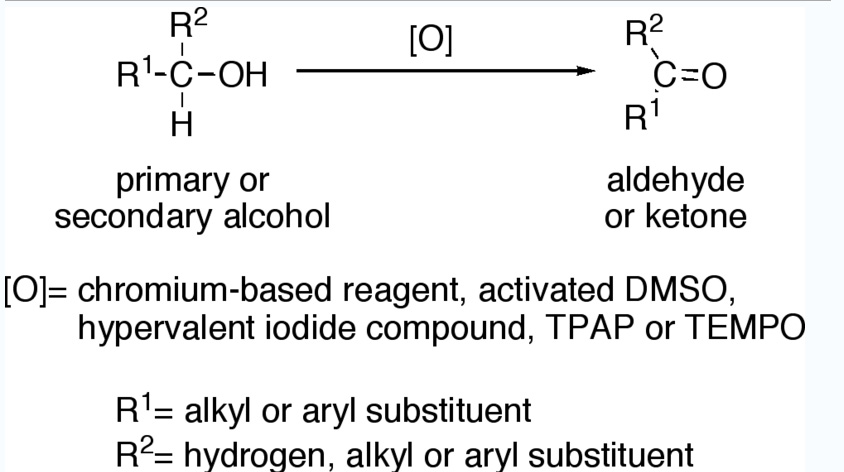
Primary alcohols (R-CH2-OH) can be oxidized either to aldehydes (R-CHO) or to carboxylic acids (R-CO2H), while the oxidation of secondary alcohols (R1R²CH-OH) normally terminates at the ketone (R1R²C=O) stage. Tertiary alcohols (R1R²R³C-OH) are resistant to oxidation.
The direct oxidation of primary alcohols to carboxylic acids normally proceeds via the corresponding aldehyde, which is transformed via an aldehyde hydrate (R-CH(OH)2) by reaction with water before it can be further oxidized to the carboxylic acid.
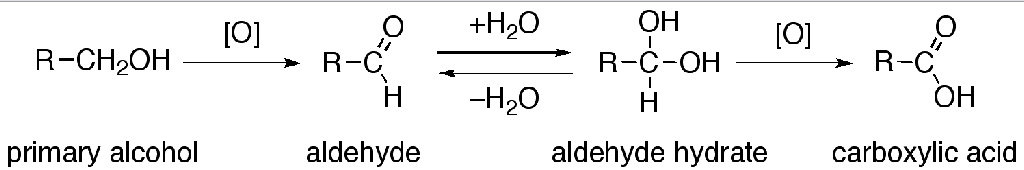
Often it is possible to interrupt the oxidation of a primary alcohol at the aldehyde level by performing the reaction in absence of water, so that no aldehyde hydrate can be formed.
Reagents useful for the transformation of primary alcohols to aldehydes are normally also suitable for the oxidation of secondary alcohols to ketones. These include:
- Chromium-based reagents, such as Collins reagent (CrO3·Py2), PDC or PCC.
- Activated DMSO, resulting from reaction of DMSO with electrophiles, such as oxalyl chloride (Swern oxidation), a carbodiimide (Pfitzner-Moffatt oxidation) or the complex SO3·Py (Parikh-Doering oxidation).
- Hypervalent iodine compounds, such as Dess-Martin periodinane or 2-Iodoxybenzoic acid.
- Catalytic TPAP in presence of excess of NMO (Ley oxidation).
- Catalytic TEMPO in presence of excess bleach (NaOCl) (Anelli’s oxidation).
Allylic and benzylic alcohols can be oxidized in presence of other alcohols using certain selective oxidants such as manganese dioxide (MnO2).
Reagents useful for the oxidation of secondary alcohols to ketones, but normally inefficient for oxidation of primary alcohols to aldehydes, include chromium trioxide (CrO3) in a mixture of sulfuric acid and acetone (Jones oxidation) and certain ketones, such as cyclohexanone, in the presence of aluminium isopropoxide (Oppenauer oxidation).
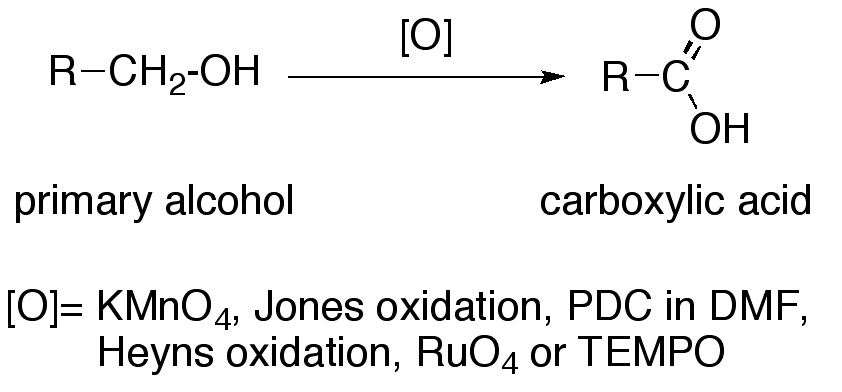
The direct oxidation of primary alcohols to carboxylic acids can be carried out using:
- Potassium permanganate (KMnO4).
- Jones oxidation.
- PDC in DMF.
- Heyns oxidation.
- Ruthenium tetroxide (RuO4).
- TEMPO.
Alcohols possessing two hydroxy groups located on adjacent carbons —that is, 1,2-diols— suffer oxidative breakage at a carbon-carbon bond with some oxidants such as sodium periodate (NaIO4) or lead tetraacetate (Pb(OAc)4), resulting in generation of two carbonyl groups.
Toxicity
Alcohols often have an odor described as 'biting' that 'hangs' in the nasal passages. Ethanol in the form of alcoholic beverages has been consumed by humans since pre-historic times, for a variety of hygienic, dietary, medicinal, religious, and recreational reasons. The consumption of large doses result in drunkenness or intoxication (which may lead to a hangover as the effect wears off) and, depending on the dose and regularity of use, can cause acute respiratory failure or death and with chronic use has medical repercussions. Because alcohol impairs judgment, it can often be a catalyst for reckless or irresponsible behavior. The LD50 of ethanol in rats is 11,300 mg/kg.[5] This ratio would correspond to an 80kg (176.4lb) man drinking 65 shots of 80 proof alcohol, although the LD50 does not necessarily translate directly to humans.
Other alcohols are substantially more poisonous than ethanol, partly because they take much longer to be metabolized, and often their metabolism produces even more toxic substances. Methanol, or wood alcohol, for instance, is oxidized by alcohol dehydrogenase enzymes in the liver to the poisonous formaldehyde, which can cause blindness or death.[2]
An effective treatment to prevent formaldehyde toxicity after methanol ingestion is to administer ethanol. Alcohol dehydrogenase has a higher affinity for ethanol, thus preventing methanol from binding and acting as a substrate. Any remaining methanol will then have time to be excreted through the kidneys. Remaining formaldehyde will be converted to formic acid and excreted.
See also
- Alcoholism
- Alcohol fuel
- Alcoholic beverage
- Blood alcohol content
- Effects of alcohol on the body
- Fatty alcohol
- Fetal alcohol syndrome
- Oxidation of primary alcohols to carboxylic acids
- Rubbing alcohol
- Sugar alcohol
- Transesterification
References
- ↑ 1.0 1.1 Lodgsdon, J.E. (1994). "Ethanol." In J.I. Kroschwitz (Ed.) Encyclopedia of Chemical Technology, 4th ed. vol. 9, p. 820. New York: John Wiley & Sons.
- ↑ 2.0 2.1 "Methanol and Blindness". Ask A Scientist, Chemistry Archive. Unknown parameter
|accessdaymonth=ignored (help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help) - ↑ 3.0 3.1 William Reusch. "Alcohols". VirtualText of Organic Chemistry. Retrieved 2007-09-14.
- ↑ "Paracelsus, Philippus Aureolus (1493–1541)". The Free Dictionary. Farlex. Retrieved 2007-09-29.
- ↑ Robert S. Gable (2004). "Comparison of acute lethal toxicity of commonly abused psychoactive substances" (reprint). Addiction. 99 (6): 686–696. doi:10.1111/j.1360-0443.2004.00744.x.
- Pages with citations using unsupported parameters
- All articles with unsourced statements
- Articles with unsourced statements from September 2007
- Articles with invalid date parameter in template
- Articles containing Arabic-language text
- Articles with unsourced statements from February 2007
- Alcohols
- Antiseptics
- Functional groups