ALDH2: Difference between revisions
m (Robot: Automated text replacement (-{{WikiDoc Cardiology Network Infobox}} +, -<references /> +{{reflist|2}}, -{{reflist}} +{{reflist|2}})) |
m (Bot: HTTP→HTTPS (v470)) |
||
| Line 1: | Line 1: | ||
< | {{Infobox_gene}} | ||
{{ | '''Aldehyde dehydrogenase, mitochondrial''' is an [[enzyme]] that in humans is encoded by the ''ALDH2'' [[gene]] located on [[chromosome 12]].<ref name="pmid4015823">{{cite journal | vauthors = Yoshida A, Ikawa M, Hsu LC, Tani K | title = Molecular abnormality and cDNA cloning of human aldehyde dehydrogenases | journal = Alcohol | volume = 2 | issue = 1 | pages = 103–6 | year = 1985 | pmid = 4015823 | doi = 10.1016/0741-8329(85)90024-2 }}</ref><ref name="pmid2987944">{{cite journal | vauthors = Hsu LC, Tani K, Fujiyoshi T, Kurachi K, Yoshida A | title = Cloning of cDNAs for human aldehyde dehydrogenases 1 and 2 | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 82 | issue = 11 | pages = 3771–5 | date = Jun 1985 | pmid = 2987944 | pmc = 397869 | doi = 10.1073/pnas.82.11.3771 | url = http://www.pnas.org/content/82/11/3771.abstract }}</ref> This protein belongs to the [[aldehyde dehydrogenase]] family of enzymes. Aldehyde dehydrogenase is the second enzyme of the major [[oxidative]] pathway of [[alcohol]] metabolism. Two major liver isoforms of aldehyde dehydrogenase, [[cytosolic]] and [[mitochondria]]l, can be distinguished by their [[electrophoretic]] mobilities, [[kinetic energy|kinetic]] properties, and [[subcellular localization]]s.<ref name ="entrez" >{{cite web | title = Entrez Gene: ALDH2 aldehyde dehydrogenase 2 family (mitochondrial)| url = https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=217| accessdate = }}</ref> | ||
| | |||
| | |||
| | |||
| | |||
| | |||
}} | |||
Most [[Caucasian race|caucasians]] have two major isozymes, while approximately 50% of East Asians have the cytosolic isozyme but not the mitochondrial isozyme. A remarkably higher frequency of acute alcohol intoxication among East Asians than among Caucasians could be related to the absence of a [[catalytic]]ally active form of the mitochondrial isozyme. The increased exposure to acetaldehyde in individuals with the catalytically inactive form may also confer greater susceptibility to many types of [[cancer]].<ref>{{cite journal | vauthors = Seitz HK, Meier P | title = The role of acetaldehyde in upper digestive tract cancer in alcoholics | journal = Transl Res | volume = 149 | issue = 6 | pages = 293–7 | year = 2007 | pmid = 17543846 | doi = 10.1016/j.trsl.2006.12.002 }}</ref> | |||
}} | |||
== Gene == | |||
The ALDH2 gene is about 44 kbp in length and contains at least 13 [[exons]] which encode 517 [[amino acid]] residues. Except for the [[signal peptide|signal NH2-terminal peptide]], which is absent in the mature enzyme, the amino acid sequence deduced from the exons coincided with the reported [[primary structure]] of human liver ALDH2. Several introns contain [[Alu element|Alu]] repetitive sequences. A [[TATA box|TATA]]-like sequence (TTATAAAA) and a [[CAAT box|CAAT]]-like sequence (GTCATCAT) are located 473 and 515 bp, respectively, upstream from the translation initiation [[codon]].<ref>{{cite journal | vauthors = Hsu LC, Bendel RE, Yoshida A | title = Genomic structure of the human mitochondrial aldehyde dehydrogenase gene | journal = Genomics | volume = 2 | issue = 1 | pages = 57–65 | date = Jan 1988 | pmid = 2838413 | doi=10.1016/0888-7543(88)90109-7}}</ref> | |||
{{clear|left}} | |||
== Enzyme structure == | |||
{{ | The enzyme encoded by the human ALDH2 gene is a tetrameric enzyme that contains three domains; two dinucleotide-binding domains and a three-stranded beta-sheet domain. The active site of ALDH2 is divided into two halves by the nicotinamide ring of NAD<sup>+</sup>. Adjacent to the A-side (Pro-R) of the [[nicotinamide]] ring is a cluster of three [[cysteine]]s (Cys301, Cys302 and Cys303) and adjacent to the B-side (Pro-S) are Thr244, Glu268, Glu476 and an ordered water molecule bound to Thr244 and Glu476.<ref>{{cite journal | vauthors = González-Segura L, Ho KK, Perez-Miller S, Weiner H, Hurley TD | title = Catalytic contribution of threonine 244 in human ALDH2 | journal = Chemico-Biological Interactions | volume = 202 | issue = 1–3 | pages = 32–40 | date = Feb 2013 | pmid = 23295226 | doi = 10.1016/j.cbi.2012.12.009 | pmc=3602351}}</ref> Although there is a recognizable [[Rossmann fold]], the coenzyme-binding region of ALDH2 binds NAD<sup>+</sup> in a manner not seen in other NAD<sup>+</sup>-binding enzymes. The positions of the residues near the nicotinamide ring of NAD<sup>+</sup> suggest a chemical mechanism whereby Glu268 functions as a general base through a bound water molecule. The sidechain amide nitrogen of Asn169 and the peptide nitrogen of Cys302 are in position to stabilize the oxyanion present in the tetrahedral transition state prior to [[hydride]] transfer. The functional importance of residue Glu487 now appears to be due to indirect interactions of this residue with the substrate-binding site via Arg264 and Arg475.<ref>{{cite journal | vauthors = Steinmetz CG, Xie P, Weiner H, Hurley TD | title = Structure of mitochondrial aldehyde dehydrogenase: the genetic component of ethanol aversion | journal = Structure | volume = 5 | issue = 5 | pages = 701–11 | date = May 1997 | pmid = 9195888 | doi=10.1016/s0969-2126(97)00224-4}}</ref> | ||
| | |||
| | |||
== Isoforms == | |||
Two major liver [[isoform]]s of this enzyme, [[cytosol]]ic and [[mitochondrion|mitochondrial]], can be distinguished by their [[Electrophoresis|electrophoretic]] mobilities, kinetic properties, and subcellular localizations. The ALDH2 gene encodes a mitochondrial isoform, which has a low [[Michaelis-Menten kinetics#Michaelis constant Km|K<sub>m</sub>]] for [[acetaldehyde]]s, and is localized in mitochondrial matrix; in contrast the ALDH1 gene codes for the cytosolic isoform.<ref name ="entrez" /> | |||
== Function == | |||
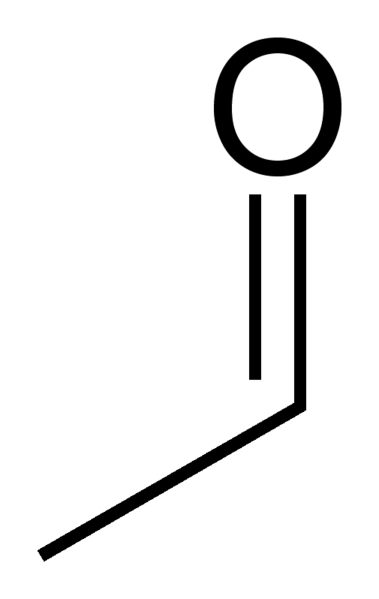
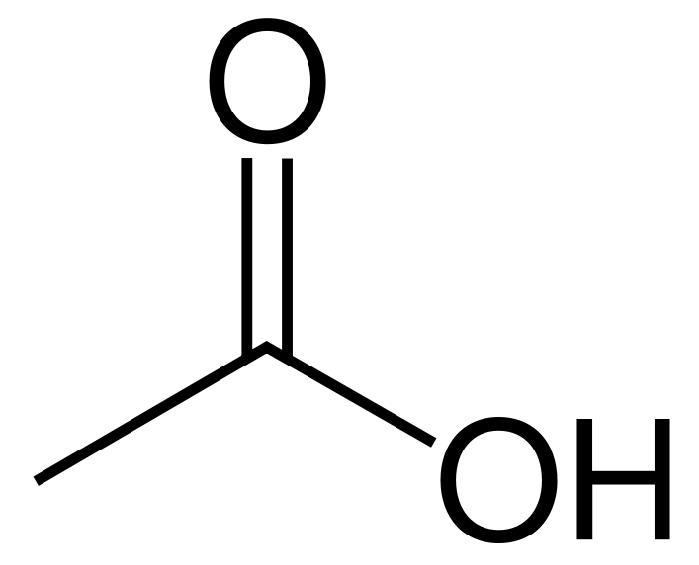
Mitochondrial aldehyde dehydrogenase belongs to the [[aldehyde dehydrogenase]] family of enzymes that catalyze the chemical transformation from [[acetaldehyde]] to [[acetic acid]]. Aldehyde dehydrogenase is the second enzyme of the major oxidative pathway of alcohol metabolism. Additionally, ALDH2 functions as a protector against oxidative stress.<ref>{{cite journal | vauthors = Ohta S, Ohsawa I, Kamino K, Ando F, Shimokata H | title = Mitochondrial ALDH2 deficiency as an oxidative stress | journal = Annals of the New York Academy of Sciences | volume = 1011 | pages = 36–44 | date = Apr 2004 | pmid = 15126281 | doi=10.1196/annals.1293.004}}</ref> | |||
<gallery> | <gallery> | ||
Image:Acetaldehyde-skeletal.svg|[[Acetaldehyde]] | |||
Image:Acetic-acid-2D-skeletal.svg|[[Acetic acid]] | |||
Image:Alda-1.svg|Chemical structure of the ALDH2 activator '''Alda-1'''. | |||
</gallery> | </gallery> | ||
[[File:Ethanol metabolism.svg|thumb|left|upright=2|alt=Ethanol metabolism in humans|Ethanol metabolism in humans]]{{clear left}} | |||
== Clinical significance == | |||
==See also== | Most Caucasians have two major isozymes, while approximately 50% of East Asians have one normal copy of the ALDH2 gene and one variant copy that encodes an inactive mitochondrial isoenzyme. In native Japanese, this variant ALDH2 gene encodes [[lysine]] instead of [[glutamic acid]] at [[amino acid]] 487 and therefore encodes a product protein that is completely inactive in metabolizing acetaldehyde to acetic acid.<ref name="ReferenceA">{{cite journal | vauthors = Takao A, Shimoda T, Kohno S, Asai S, Harda S | title = Correlation between alcohol-induced asthma and acetaldehyde dehydrogenase-2 genotype | journal = The Journal of Allergy and Clinical Immunology | volume = 101 | issue = 5 | pages = 576–80 | date = May 1998 | pmid = 9600491 | doi = 10.1016/S0091-6749(98)70162-9 }}</ref> In the overall Japanese population, about 57% of individuals are [[homozygous]] for the normal gene, 40% are [[heterozygous]] for the variant gene, and 3% are homozygous for the variant gene.<ref name="ReferenceA"/> Since ALDH2 assembles and functions as a [[tetramer]] and requires all four of its components to be active in order to metabolize acetaldehyde, heterozygotes have very little ALDH2 activity.<ref>{{cite journal | vauthors = Koppaka V, Thompson DC, Chen Y, Ellermann M, Nicolaou KC, Juvonen RO, Petersen D, Deitrich RA, Hurley TD, Vasiliou V | title = Aldehyde dehydrogenase inhibitors: a comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application | journal = Pharmacological Reviews | volume = 64 | issue = 3 | pages = 520–39 | date = Jul 2012 | pmid = 22544865 | doi = 10.1124/pr.111.005538 | pmc=3400832}}</ref> Accordingly, individuals heterozygous or homozygous for the abnormal gene metabolize ethanol to acetaldehyde normally but metabolize acetaldehyde poorly and are thereby susceptible to certain adverse effects of alcoholic (i.e. ethanol-containing) beverages; these effects include the transient accumulation of acetaldehyde in blood and tissues; facial [[flushing (physiology)|flushing]] (i.e. the "oriental flushing syndrome"), [[urticaria]], systemic [[dermatitis]], and [[alcohol-induced respiratory reactions]] such as [[rhinitis]] and the exacerbation of [[asthma]] [[bronchoconstriction]].<ref name="Ann Allergy Asthma Immunol 2013">{{cite journal | vauthors = Adams KE, Rans TS | title = Adverse reactions to alcohol and alcoholic beverages | journal = Annals of Allergy, Asthma & Immunology | volume = 111 | issue = 6 | pages = 439–45 | date = Dec 2013 | pmid = 24267355 | doi = 10.1016/j.anai.2013.09.016 }}</ref> The cited allergic reaction-like symptoms: a) do not appear due to classical [[IgE]] or [[T cell]]-related [[allergen]]-induced reactions but rather to the actions of acetaldehyde in stimulating the release of [[histamine]], a probable mediating cause of these symptoms; b) typically occur within 30–60 minutes of ingesting alcoholic beverages; and c) occur in other Asian as well as non-Asian individuals that are either seriously defective in metabolizing ingested ethanol past acetaldehyde to acetic acid or, alternatively, that metabolize ethanol too rapidly for ALDH2 processing.<ref name="Ann Allergy Asthma Immunol 2013"/><ref>{{cite journal | vauthors = Linneberg A, Gonzalez-Quintela A, Vidal C, Jørgensen T, Fenger M, Hansen T, Pedersen O, Husemoen LL | title = Genetic determinants of both ethanol and acetaldehyde metabolism influence alcohol hypersensitivity and drinking behaviour among Scandinavians | journal = Clinical and Experimental Allergy | volume = 40 | issue = 1 | pages = 123–30 | date = Jan 2010 | pmid = 20205700 | doi = 10.1111/j.1365-2222.2009.03398.x }}</ref> | ||
A remarkably higher frequency of acute alcohol intoxication among East Asians than among Caucasians has been repeatedly shown to be related to the very much reduced activity of the variant ALDH2-2 isoenzyme.<ref name ="entrez"/> During the 80's there has been a steady increase in the number of Japanese alcoholics who manage to overcome their genetically determined aversion to [[alcoholism]] from the dominant effects of an ALDH2-2 mutation.<ref name="pmid7907720">{{cite journal | vauthors = Higuchi S, Matsushita S, Imazeki H, Kinoshita T, Takagi S, Kono H | title = Aldehyde dehydrogenase genotypes in Japanese alcoholics | journal = Lancet | volume = 343 | issue = 8899 | pages = 741–2 | date = Mar 1994 | pmid = 7907720 | doi = 10.1016/S0140-6736(94)91629-2 }}</ref> This trend demonstrates that, even among those least likely to succumb to alcoholism, there are social pressures to drink.<ref name="pmid7907720"/> | |||
An activator of ALDH2 enzymatic activity, Alda-1 (N-(1,3-benzodioxol-5-ylmethyl)-2,6-dichlorobenzamide), has been shown to reduce [[ischemia]]-induced cardiac damage caused by [[myocardial infarction]].<ref name="Chen_2008">{{cite journal | vauthors = Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D | title = Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart | journal = Science | volume = 321 | issue = 5895 | pages = 1493–5 | date = Sep 2008 | pmid = 18787169 | pmc = 2741612 | doi = 10.1126/science.1158554 }}</ref> | |||
== Interactions == | |||
ALDH2 has been shown to [[Protein-protein interaction|interact]] with [[GroEL]].<ref name="pmid12387818">{{cite journal | vauthors = Lee KH, Kim HS, Jeong HS, Lee YS | title = Chaperonin GroESL mediates the protein folding of human liver mitochondrial aldehyde dehydrogenase in Escherichia coli | journal = Biochemical and Biophysical Research Communications | volume = 298 | issue = 2 | pages = 216–24 | date = Oct 2002 | pmid = 12387818 | doi = 10.1016/S0006-291X(02)02423-3 }}</ref> | |||
== See also == | |||
* [[Acetaldehyde dehydrogenase]] | * [[Acetaldehyde dehydrogenase]] | ||
* [[Alcohol dehydrogenase]] | |||
* [[Alcohol flush reaction]] | |||
* [[Alcohol-induced respiratory reactions]] | |||
==References== | == References == | ||
{{ | {{Reflist|33em}} | ||
==Further reading== | {{Clear}} | ||
{{refbegin | | |||
== Further reading == | |||
{{refbegin|33em}} | |||
*{{cite journal | * {{cite journal | vauthors = Yoshida A | title = Molecular genetics of human aldehyde dehydrogenase | journal = Pharmacogenetics | volume = 2 | issue = 4 | pages = 139–47 | year = 1992 | pmid = 1306115 | doi = 10.1097/00008571-199208000-00001 }} | ||
*{{cite journal | * {{cite journal | vauthors = Chao YC, Liou SR, Tsai SF, Yin SJ | title = Dominance of the mutant ALDH2(2) allele in the expression of human stomach aldehyde dehydrogenase-2 activity | journal = Proc. Natl. Sci. Counc. Repub. China B | volume = 17 | issue = 3 | pages = 98–102 | year = 1993 | pmid = 8290656 | doi = }} | ||
*{{cite journal | * {{cite journal | vauthors = Crabb DW, Edenberg HJ, Bosron WF, Li TK | title = Genotypes for aldehyde dehydrogenase deficiency and alcohol sensitivity. The inactive ALDH2(2) allele is dominant | journal = J. Clin. Invest. | volume = 83 | issue = 1 | pages = 314–6 | year = 1989 | pmid = 2562960 | pmc = 303676 | doi = 10.1172/JCI113875 }} | ||
* {{cite journal | vauthors = Hsu LC, Bendel RE, Yoshida A | title = Genomic structure of the human mitochondrial aldehyde dehydrogenase gene | journal = Genomics | volume = 2 | issue = 1 | pages = 57–65 | year = 1988 | pmid = 2838413 | doi = 10.1016/0888-7543(88)90109-7 }} | |||
*{{cite journal | * {{cite journal | vauthors = Hsu LC, Tani K, Fujiyoshi T, Kurachi K, Yoshida A | title = Cloning of cDNAs for human aldehyde dehydrogenases 1 and 2 | journal = Proc. Natl. Acad. Sci. U.S.A. | volume = 82 | issue = 11 | pages = 3771–5 | year = 1985 | pmid = 2987944 | pmc = 397869 | doi = 10.1073/pnas.82.11.3771 }} | ||
*{{cite journal | * {{cite journal | vauthors = Braun T, Grzeschik KH, Bober E, Singh S, Agarwal DP, Goedde HW | title = The structural gene for the mitochondrial aldehyde dehydrogenase maps to human chromosome 12 | journal = Hum. Genet. | volume = 73 | issue = 4 | pages = 365–7 | year = 1986 | pmid = 3017845 | doi = 10.1007/BF00279102 }} | ||
*{{cite journal | * {{cite journal | vauthors = Braun T, Bober E, Singh S, Agarwal DP, Goedde HW | title = Isolation and sequence analysis of a full length cDNA clone coding for human mitochondrial aldehyde dehydrogenase | journal = Nucleic Acids Res. | volume = 15 | issue = 7 | pages = 3179 | year = 1987 | pmid = 3562250 | pmc = 340920 | doi = 10.1093/nar/15.7.3179 }} | ||
*{{cite journal | * {{cite journal | vauthors = Braun T, Bober E, Singh S, Agarwal DP, Goedde HW | title = Evidence for a signal peptide at the amino-terminal end of human mitochondrial aldehyde dehydrogenase | journal = FEBS Lett. | volume = 215 | issue = 2 | pages = 233–6 | year = 1987 | pmid = 3582651 | doi = 10.1016/0014-5793(87)80152-7 }} | ||
*{{cite journal | * {{cite journal | vauthors = Agarwal DP, Goedde HW | title = Human aldehyde dehydrogenase isozymes and alcohol sensitivity | journal = Isozymes Curr. Top. Biol. Med. Res. | volume = 16 | issue = | pages = 21–48 | year = 1987 | pmid = 3610592 | doi = }} | ||
*{{cite journal | * {{cite journal | vauthors = Hempel J, Höög JO, Jörnvall H | title = Mitochondrial aldehyde dehydrogenase. Homology of putative targeting sequence to that of carbamyl phosphate synthetase I revealed by correlation of cDNA and protein data | journal = FEBS Lett. | volume = 222 | issue = 1 | pages = 95–8 | year = 1987 | pmid = 3653404 | doi = 10.1016/0014-5793(87)80198-9 }} | ||
*{{cite journal | * {{cite journal | vauthors = Yoshida A, Ikawa M, Hsu LC, Tani K | title = Molecular abnormality and cDNA cloning of human aldehyde dehydrogenases | journal = Alcohol | volume = 2 | issue = 1 | pages = 103–6 | year = 1985 | pmid = 4015823 | doi = 10.1016/0741-8329(85)90024-2 }} | ||
*{{cite journal | * {{cite journal | vauthors = Hempel J, Kaiser R, Jörnvall H | title = Mitochondrial aldehyde dehydrogenase from human liver. Primary structure, differences in relation to the cytosolic enzyme, and functional correlations | journal = Eur. J. Biochem. | volume = 153 | issue = 1 | pages = 13–28 | year = 1985 | pmid = 4065146 | doi = 10.1111/j.1432-1033.1985.tb09260.x }} | ||
*{{cite journal | * {{cite journal | vauthors = Yoshida A, Huang IY, Ikawa M | title = Molecular abnormality of an inactive aldehyde dehydrogenase variant commonly found in Orientals | journal = Proc. Natl. Acad. Sci. U.S.A. | volume = 81 | issue = 1 | pages = 258–61 | year = 1984 | pmid = 6582480 | pmc = 344651 | doi = 10.1073/pnas.81.1.258 }} | ||
*{{cite journal | * {{cite journal | vauthors = Xiao Q, Weiner H, Johnston T, Crabb DW | title = The aldehyde dehydrogenase ALDH2*2 allele exhibits dominance over ALDH2*1 in transduced HeLa cells | journal = J. Clin. Invest. | volume = 96 | issue = 5 | pages = 2180–6 | year = 1995 | pmid = 7593603 | pmc = 185867 | doi = 10.1172/JCI118272 }} | ||
*{{cite journal | * {{cite journal | vauthors = Maruyama K, Sugano S | title = Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides | journal = Gene | volume = 138 | issue = 1–2 | pages = 171–4 | year = 1994 | pmid = 8125298 | doi = 10.1016/0378-1119(94)90802-8 }} | ||
*{{cite journal | * {{cite journal | vauthors = Novoradovsky A, Tsai SJ, Goldfarb L, Peterson R, Long JC, Goldman D | title = Mitochondrial aldehyde dehydrogenase polymorphism in Asian and American Indian populations: detection of new ALDH2 alleles | journal = Alcohol. Clin. Exp. Res. | volume = 19 | issue = 5 | pages = 1105–10 | year = 1995 | pmid = 8561277 | doi = 10.1111/j.1530-0277.1995.tb01587.x }} | ||
*{{cite journal | * {{cite journal | vauthors = Xiao Q, Weiner H, Crabb DW | title = The mutation in the mitochondrial aldehyde dehydrogenase (ALDH2) gene responsible for alcohol-induced flushing increases turnover of the enzyme tetramers in a dominant fashion | journal = J. Clin. Invest. | volume = 98 | issue = 9 | pages = 2027–32 | year = 1996 | pmid = 8903321 | pmc = 507646 | doi = 10.1172/JCI119007 }} | ||
*{{cite journal | |||
}} | |||
{{refend}} | {{refend}} | ||
==External links== | == External links == | ||
* {{ | {{Commons category|ALDH2}} | ||
* {{MeSH name|ALDH2 protein, human}} | |||
* {{UCSC gene info|ALDH2}} | |||
{{ | {{PDB Gallery|geneid=217}} | ||
{{Aldehyde | {{Aldehyde dehydrogenases}} | ||
{{Mitochondrial enzymes}} | {{Mitochondrial enzymes}} | ||
{{Enzymes}} | |||
{{Portal bar|Molecular and Cellular Biology|border=no}} | |||
[[Category:EC 1.2.1]] | [[Category:EC 1.2.1]] | ||
[[Category:Genes on human chromosome 12]] | |||
Revision as of 01:36, 27 October 2017
| VALUE_ERROR (nil) | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Aliases | |||||||
| External IDs | GeneCards: [1] | ||||||
| Orthologs | |||||||
| Species | Human | Mouse | |||||
| Entrez |
|
| |||||
| Ensembl |
|
| |||||
| UniProt |
|
| |||||
| RefSeq (mRNA) |
|
| |||||
| RefSeq (protein) |
|
| |||||
| Location (UCSC) | n/a | n/a | |||||
| PubMed search | n/a | n/a | |||||
| Wikidata | |||||||
| |||||||
Aldehyde dehydrogenase, mitochondrial is an enzyme that in humans is encoded by the ALDH2 gene located on chromosome 12.[1][2] This protein belongs to the aldehyde dehydrogenase family of enzymes. Aldehyde dehydrogenase is the second enzyme of the major oxidative pathway of alcohol metabolism. Two major liver isoforms of aldehyde dehydrogenase, cytosolic and mitochondrial, can be distinguished by their electrophoretic mobilities, kinetic properties, and subcellular localizations.[3]
Most caucasians have two major isozymes, while approximately 50% of East Asians have the cytosolic isozyme but not the mitochondrial isozyme. A remarkably higher frequency of acute alcohol intoxication among East Asians than among Caucasians could be related to the absence of a catalytically active form of the mitochondrial isozyme. The increased exposure to acetaldehyde in individuals with the catalytically inactive form may also confer greater susceptibility to many types of cancer.[4]
Gene
The ALDH2 gene is about 44 kbp in length and contains at least 13 exons which encode 517 amino acid residues. Except for the signal NH2-terminal peptide, which is absent in the mature enzyme, the amino acid sequence deduced from the exons coincided with the reported primary structure of human liver ALDH2. Several introns contain Alu repetitive sequences. A TATA-like sequence (TTATAAAA) and a CAAT-like sequence (GTCATCAT) are located 473 and 515 bp, respectively, upstream from the translation initiation codon.[5]
Enzyme structure
The enzyme encoded by the human ALDH2 gene is a tetrameric enzyme that contains three domains; two dinucleotide-binding domains and a three-stranded beta-sheet domain. The active site of ALDH2 is divided into two halves by the nicotinamide ring of NAD+. Adjacent to the A-side (Pro-R) of the nicotinamide ring is a cluster of three cysteines (Cys301, Cys302 and Cys303) and adjacent to the B-side (Pro-S) are Thr244, Glu268, Glu476 and an ordered water molecule bound to Thr244 and Glu476.[6] Although there is a recognizable Rossmann fold, the coenzyme-binding region of ALDH2 binds NAD+ in a manner not seen in other NAD+-binding enzymes. The positions of the residues near the nicotinamide ring of NAD+ suggest a chemical mechanism whereby Glu268 functions as a general base through a bound water molecule. The sidechain amide nitrogen of Asn169 and the peptide nitrogen of Cys302 are in position to stabilize the oxyanion present in the tetrahedral transition state prior to hydride transfer. The functional importance of residue Glu487 now appears to be due to indirect interactions of this residue with the substrate-binding site via Arg264 and Arg475.[7]
Isoforms
Two major liver isoforms of this enzyme, cytosolic and mitochondrial, can be distinguished by their electrophoretic mobilities, kinetic properties, and subcellular localizations. The ALDH2 gene encodes a mitochondrial isoform, which has a low Km for acetaldehydes, and is localized in mitochondrial matrix; in contrast the ALDH1 gene codes for the cytosolic isoform.[3]
Function
Mitochondrial aldehyde dehydrogenase belongs to the aldehyde dehydrogenase family of enzymes that catalyze the chemical transformation from acetaldehyde to acetic acid. Aldehyde dehydrogenase is the second enzyme of the major oxidative pathway of alcohol metabolism. Additionally, ALDH2 functions as a protector against oxidative stress.[8]
-
Chemical structure of the ALDH2 activator Alda-1.
Clinical significance
Most Caucasians have two major isozymes, while approximately 50% of East Asians have one normal copy of the ALDH2 gene and one variant copy that encodes an inactive mitochondrial isoenzyme. In native Japanese, this variant ALDH2 gene encodes lysine instead of glutamic acid at amino acid 487 and therefore encodes a product protein that is completely inactive in metabolizing acetaldehyde to acetic acid.[9] In the overall Japanese population, about 57% of individuals are homozygous for the normal gene, 40% are heterozygous for the variant gene, and 3% are homozygous for the variant gene.[9] Since ALDH2 assembles and functions as a tetramer and requires all four of its components to be active in order to metabolize acetaldehyde, heterozygotes have very little ALDH2 activity.[10] Accordingly, individuals heterozygous or homozygous for the abnormal gene metabolize ethanol to acetaldehyde normally but metabolize acetaldehyde poorly and are thereby susceptible to certain adverse effects of alcoholic (i.e. ethanol-containing) beverages; these effects include the transient accumulation of acetaldehyde in blood and tissues; facial flushing (i.e. the "oriental flushing syndrome"), urticaria, systemic dermatitis, and alcohol-induced respiratory reactions such as rhinitis and the exacerbation of asthma bronchoconstriction.[11] The cited allergic reaction-like symptoms: a) do not appear due to classical IgE or T cell-related allergen-induced reactions but rather to the actions of acetaldehyde in stimulating the release of histamine, a probable mediating cause of these symptoms; b) typically occur within 30–60 minutes of ingesting alcoholic beverages; and c) occur in other Asian as well as non-Asian individuals that are either seriously defective in metabolizing ingested ethanol past acetaldehyde to acetic acid or, alternatively, that metabolize ethanol too rapidly for ALDH2 processing.[11][12]
A remarkably higher frequency of acute alcohol intoxication among East Asians than among Caucasians has been repeatedly shown to be related to the very much reduced activity of the variant ALDH2-2 isoenzyme.[3] During the 80's there has been a steady increase in the number of Japanese alcoholics who manage to overcome their genetically determined aversion to alcoholism from the dominant effects of an ALDH2-2 mutation.[13] This trend demonstrates that, even among those least likely to succumb to alcoholism, there are social pressures to drink.[13]
An activator of ALDH2 enzymatic activity, Alda-1 (N-(1,3-benzodioxol-5-ylmethyl)-2,6-dichlorobenzamide), has been shown to reduce ischemia-induced cardiac damage caused by myocardial infarction.[14]
Interactions
ALDH2 has been shown to interact with GroEL.[15]
See also
- Acetaldehyde dehydrogenase
- Alcohol dehydrogenase
- Alcohol flush reaction
- Alcohol-induced respiratory reactions
References
- ↑ Yoshida A, Ikawa M, Hsu LC, Tani K (1985). "Molecular abnormality and cDNA cloning of human aldehyde dehydrogenases". Alcohol. 2 (1): 103–6. doi:10.1016/0741-8329(85)90024-2. PMID 4015823.
- ↑ Hsu LC, Tani K, Fujiyoshi T, Kurachi K, Yoshida A (Jun 1985). "Cloning of cDNAs for human aldehyde dehydrogenases 1 and 2". Proceedings of the National Academy of Sciences of the United States of America. 82 (11): 3771–5. doi:10.1073/pnas.82.11.3771. PMC 397869. PMID 2987944.
- ↑ 3.0 3.1 3.2 "Entrez Gene: ALDH2 aldehyde dehydrogenase 2 family (mitochondrial)".
- ↑ Seitz HK, Meier P (2007). "The role of acetaldehyde in upper digestive tract cancer in alcoholics". Transl Res. 149 (6): 293–7. doi:10.1016/j.trsl.2006.12.002. PMID 17543846.
- ↑ Hsu LC, Bendel RE, Yoshida A (Jan 1988). "Genomic structure of the human mitochondrial aldehyde dehydrogenase gene". Genomics. 2 (1): 57–65. doi:10.1016/0888-7543(88)90109-7. PMID 2838413.
- ↑ González-Segura L, Ho KK, Perez-Miller S, Weiner H, Hurley TD (Feb 2013). "Catalytic contribution of threonine 244 in human ALDH2". Chemico-Biological Interactions. 202 (1–3): 32–40. doi:10.1016/j.cbi.2012.12.009. PMC 3602351. PMID 23295226.
- ↑ Steinmetz CG, Xie P, Weiner H, Hurley TD (May 1997). "Structure of mitochondrial aldehyde dehydrogenase: the genetic component of ethanol aversion". Structure. 5 (5): 701–11. doi:10.1016/s0969-2126(97)00224-4. PMID 9195888.
- ↑ Ohta S, Ohsawa I, Kamino K, Ando F, Shimokata H (Apr 2004). "Mitochondrial ALDH2 deficiency as an oxidative stress". Annals of the New York Academy of Sciences. 1011: 36–44. doi:10.1196/annals.1293.004. PMID 15126281.
- ↑ 9.0 9.1 Takao A, Shimoda T, Kohno S, Asai S, Harda S (May 1998). "Correlation between alcohol-induced asthma and acetaldehyde dehydrogenase-2 genotype". The Journal of Allergy and Clinical Immunology. 101 (5): 576–80. doi:10.1016/S0091-6749(98)70162-9. PMID 9600491.
- ↑ Koppaka V, Thompson DC, Chen Y, Ellermann M, Nicolaou KC, Juvonen RO, Petersen D, Deitrich RA, Hurley TD, Vasiliou V (Jul 2012). "Aldehyde dehydrogenase inhibitors: a comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application". Pharmacological Reviews. 64 (3): 520–39. doi:10.1124/pr.111.005538. PMC 3400832. PMID 22544865.
- ↑ 11.0 11.1 Adams KE, Rans TS (Dec 2013). "Adverse reactions to alcohol and alcoholic beverages". Annals of Allergy, Asthma & Immunology. 111 (6): 439–45. doi:10.1016/j.anai.2013.09.016. PMID 24267355.
- ↑ Linneberg A, Gonzalez-Quintela A, Vidal C, Jørgensen T, Fenger M, Hansen T, Pedersen O, Husemoen LL (Jan 2010). "Genetic determinants of both ethanol and acetaldehyde metabolism influence alcohol hypersensitivity and drinking behaviour among Scandinavians". Clinical and Experimental Allergy. 40 (1): 123–30. doi:10.1111/j.1365-2222.2009.03398.x. PMID 20205700.
- ↑ 13.0 13.1 Higuchi S, Matsushita S, Imazeki H, Kinoshita T, Takagi S, Kono H (Mar 1994). "Aldehyde dehydrogenase genotypes in Japanese alcoholics". Lancet. 343 (8899): 741–2. doi:10.1016/S0140-6736(94)91629-2. PMID 7907720.
- ↑ Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D (Sep 2008). "Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart". Science. 321 (5895): 1493–5. doi:10.1126/science.1158554. PMC 2741612. PMID 18787169.
- ↑ Lee KH, Kim HS, Jeong HS, Lee YS (Oct 2002). "Chaperonin GroESL mediates the protein folding of human liver mitochondrial aldehyde dehydrogenase in Escherichia coli". Biochemical and Biophysical Research Communications. 298 (2): 216–24. doi:10.1016/S0006-291X(02)02423-3. PMID 12387818.
Further reading
- Yoshida A (1992). "Molecular genetics of human aldehyde dehydrogenase". Pharmacogenetics. 2 (4): 139–47. doi:10.1097/00008571-199208000-00001. PMID 1306115.
- Chao YC, Liou SR, Tsai SF, Yin SJ (1993). "Dominance of the mutant ALDH2(2) allele in the expression of human stomach aldehyde dehydrogenase-2 activity". Proc. Natl. Sci. Counc. Repub. China B. 17 (3): 98–102. PMID 8290656.
- Crabb DW, Edenberg HJ, Bosron WF, Li TK (1989). "Genotypes for aldehyde dehydrogenase deficiency and alcohol sensitivity. The inactive ALDH2(2) allele is dominant". J. Clin. Invest. 83 (1): 314–6. doi:10.1172/JCI113875. PMC 303676. PMID 2562960.
- Hsu LC, Bendel RE, Yoshida A (1988). "Genomic structure of the human mitochondrial aldehyde dehydrogenase gene". Genomics. 2 (1): 57–65. doi:10.1016/0888-7543(88)90109-7. PMID 2838413.
- Hsu LC, Tani K, Fujiyoshi T, Kurachi K, Yoshida A (1985). "Cloning of cDNAs for human aldehyde dehydrogenases 1 and 2". Proc. Natl. Acad. Sci. U.S.A. 82 (11): 3771–5. doi:10.1073/pnas.82.11.3771. PMC 397869. PMID 2987944.
- Braun T, Grzeschik KH, Bober E, Singh S, Agarwal DP, Goedde HW (1986). "The structural gene for the mitochondrial aldehyde dehydrogenase maps to human chromosome 12". Hum. Genet. 73 (4): 365–7. doi:10.1007/BF00279102. PMID 3017845.
- Braun T, Bober E, Singh S, Agarwal DP, Goedde HW (1987). "Isolation and sequence analysis of a full length cDNA clone coding for human mitochondrial aldehyde dehydrogenase". Nucleic Acids Res. 15 (7): 3179. doi:10.1093/nar/15.7.3179. PMC 340920. PMID 3562250.
- Braun T, Bober E, Singh S, Agarwal DP, Goedde HW (1987). "Evidence for a signal peptide at the amino-terminal end of human mitochondrial aldehyde dehydrogenase". FEBS Lett. 215 (2): 233–6. doi:10.1016/0014-5793(87)80152-7. PMID 3582651.
- Agarwal DP, Goedde HW (1987). "Human aldehyde dehydrogenase isozymes and alcohol sensitivity". Isozymes Curr. Top. Biol. Med. Res. 16: 21–48. PMID 3610592.
- Hempel J, Höög JO, Jörnvall H (1987). "Mitochondrial aldehyde dehydrogenase. Homology of putative targeting sequence to that of carbamyl phosphate synthetase I revealed by correlation of cDNA and protein data". FEBS Lett. 222 (1): 95–8. doi:10.1016/0014-5793(87)80198-9. PMID 3653404.
- Yoshida A, Ikawa M, Hsu LC, Tani K (1985). "Molecular abnormality and cDNA cloning of human aldehyde dehydrogenases". Alcohol. 2 (1): 103–6. doi:10.1016/0741-8329(85)90024-2. PMID 4015823.
- Hempel J, Kaiser R, Jörnvall H (1985). "Mitochondrial aldehyde dehydrogenase from human liver. Primary structure, differences in relation to the cytosolic enzyme, and functional correlations". Eur. J. Biochem. 153 (1): 13–28. doi:10.1111/j.1432-1033.1985.tb09260.x. PMID 4065146.
- Yoshida A, Huang IY, Ikawa M (1984). "Molecular abnormality of an inactive aldehyde dehydrogenase variant commonly found in Orientals". Proc. Natl. Acad. Sci. U.S.A. 81 (1): 258–61. doi:10.1073/pnas.81.1.258. PMC 344651. PMID 6582480.
- Xiao Q, Weiner H, Johnston T, Crabb DW (1995). "The aldehyde dehydrogenase ALDH2*2 allele exhibits dominance over ALDH2*1 in transduced HeLa cells". J. Clin. Invest. 96 (5): 2180–6. doi:10.1172/JCI118272. PMC 185867. PMID 7593603.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Novoradovsky A, Tsai SJ, Goldfarb L, Peterson R, Long JC, Goldman D (1995). "Mitochondrial aldehyde dehydrogenase polymorphism in Asian and American Indian populations: detection of new ALDH2 alleles". Alcohol. Clin. Exp. Res. 19 (5): 1105–10. doi:10.1111/j.1530-0277.1995.tb01587.x. PMID 8561277.
- Xiao Q, Weiner H, Crabb DW (1996). "The mutation in the mitochondrial aldehyde dehydrogenase (ALDH2) gene responsible for alcohol-induced flushing increases turnover of the enzyme tetramers in a dominant fashion". J. Clin. Invest. 98 (9): 2027–32. doi:10.1172/JCI119007. PMC 507646. PMID 8903321.
External links
| Wikimedia Commons has media related to ALDH2. |
- ALDH2 protein, human at the US National Library of Medicine Medical Subject Headings (MeSH)
- Human ALDH2 genome location and ALDH2 gene details page in the UCSC Genome Browser.