Sotalol
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alonso Alvarado, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
INDUCED ARRHYTHMIA: To minimize the risk of induced arrhythmia, patients initiated or re-initiated on sotalol hydrochloride tablets (AF) should be placed for a minimum of three days (on their maintenance dose) in a facility that can provide cardiac resuscitation, continuous electrocardiographic monitoring and calculations of creatinine clearance. For detailed instructions regarding dose selection and special cautions for people with renal impairment. Sotalol is also indicated for the treatment of documented life-threatening ventricular arrhythmias and is marketed under the brand name Betapace (sotalol hydrochloride). Sotalol hydrochloride tablets, however, must not be substituted for Betapace AF (sotalol hydrochloride tablets, USP (AF)) because of significant differences in labeling (i.e. patient package insert, dosing administration and safety information).
|
Overview
Sotalol is a Template:Beta-adrenergic blocker, Template:Antiarrhythmic that is FDA approved for the {{{indicationType}}} of ventricular arrhythmias, symptomatic atrial fibtillation, symptomatic atriall flutter. There is a Black Box Warning for this drug as shown here. Common adverse reactions include bradyarrhythmia, chest pain, lightheadedness, palpitations, rash, nausea, dizziness, headache, dyspnea, fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
- Bronchial asthma.
- Sinus bradycardia.
- Second degree AV block and third degree AV block, unless a functioning pacemaker is present.
- Congenital or acquired long QT syndromes.
- Cardiogenic shock.
- Uncontrolled congestive heart failure.
- Hypersensitivity to Betapace.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
INDUCED ARRHYTHMIA: To minimize the risk of induced arrhythmia, patients initiated or re-initiated on sotalol hydrochloride tablets (AF) should be placed for a minimum of three days (on their maintenance dose) in a facility that can provide cardiac resuscitation, continuous electrocardiographic monitoring and calculations of creatinine clearance. For detailed instructions regarding dose selection and special cautions for people with renal impairment. Sotalol is also indicated for the treatment of documented life-threatening ventricular arrhythmias and is marketed under the brand name Betapace (sotalol hydrochloride). Sotalol hydrochloride tablets, however, must not be substituted for Betapace AF (sotalol hydrochloride tablets, USP (AF)) because of significant differences in labeling (i.e. patient package insert, dosing administration and safety information).
|
Ventricular Arrhythmia
Sotalol (AF) can cause serious ventricular arrhythmias, primarily Torsade de Pointes (TdP) type ventricular tachycardia, a polymorphic ventricular tachycardia associated with QT interval prolongation. QT interval prolongation is directly related to the dose of sotalol (AF). Factors such as reduced creatinine clearance, gender (female) and larger doses increase the risk of TdP. The risk of TdP can be reduced by adjustment of the sotalol (AF) dose according to creatinine clearance and by monitoring the ECG for excessive increases in the QT interval.
Treatment with sotalol (AF) must therefore be started only in patients observed for a minimum of three days on their maintenance dose in a facility that can provide electrocardiographic monitoring and in the presence of personnel trained in the management of serious ventricular arrhythmias. Calculation of the creatinine clearance must precede administration of the first dose of sotalol (AF). For detailed instructions regarding dose selection.
Proarrhythmia in Atrial Fibrillation/Atrial Flutter Patients
In eight controlled trials of patients with AFIB/AFL and other supraventricular arrhythmias (N=659) there were four cases of Torsade de Pointes reported (0.6%) during the controlled phase of treatment with sotalol (AF). The incidence of Torsade de Pointes was significantly lower in those patients receiving total daily doses of 320 mg or less (0.3%), as summarized in Table 5 below. Both patients who had Torsade de Pointes in the group receiving >320 mg/day were receiving 640 mg/day. In the group receiving ≤320 mg daily, one case of TdP occurred at a daily dose of 320 mg on day 4 of treatment and one case occurred on a daily dose of 160 mg on day 1 of treatment.

The table below relates the incidence of Torsade de Pointes to on-therapy QTc and change in QTc from baseline. It should be noted, however, that the highest on therapy QTc was in many cases the one obtained at the time of the Torsade de Pointes event, so that the table overstates the predictive value of a high QTc.

{clr
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Sotalol Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Sotalol Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Sotalol Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Sotalol in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Sotalol in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Sotalol during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Sotalol in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Sotalol in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Sotalol in geriatric settings.
Gender
There is no FDA guidance on the use of Sotalol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Sotalol with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Sotalol in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Sotalol in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Sotalol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Sotalol in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Sotalol Administration in the drug label.
Monitoring
There is limited information regarding Sotalol Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Sotalol and IV administrations.
Overdosage
There is limited information regarding Sotalol overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Sotalol Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Sotalol Mechanism of Action in the drug label.
Structure
There is limited information regarding Sotalol Structure in the drug label.
Pharmacodynamics
There is limited information regarding Sotalol Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Sotalol Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Sotalol Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Sotalol Clinical Studies in the drug label.
How Supplied
There is limited information regarding Sotalol How Supplied in the drug label.
Storage
There is limited information regarding Sotalol Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Sotalol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Sotalol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Sotalol Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Sotalol interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Sotalol Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Sotalol Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
In addition to dose and presence of sustained VT, other risk factors for Torsade de Pointes were gender (females had a higher incidence), excessive prolongation of the QTc interval and history of cardiomegaly or congestive heart failure. Patients with sustained ventricular tachycardia and a history of congestive heart failure appear to have the highest risk for serious proarrhythmia (7%). Of the ventricular arrhythmia patients experiencing Torsade de Pointes, approximately two-thirds spontaneously reverted to their baseline rhythm. The others were either converted electrically (D/C cardioversion or overdrive pacing) or treated with other drugs. It is not possible to determine whether some sudden deaths represented episodes of Torsade de Pointes, but in some instances sudden death did follow a documented episode of Torsade de Pointes. Although sotalol therapy was discontinued in most patients experiencing Torsade de Pointes, 17% were continued on a lower dose.
Use with Drugs that Prolong QT Interval and Antiarrhythmic Agents
The use of sotalo (AF) in conjunction with other drugs that prolong the QT interval has not been studied and is not recommended. Such drugs include many antiarrhythmics, some phenothiazines, bepridil, tricyclic antidepressants, and certain oral macrolides. Class I or Class III antiarrhythmic agents should be withheld for at least three half-lives prior to dosing with sotalol (AF). In clinical trials, sotalots (AF) was not administered to patients previously treated with oral amiodarone for >1 month in the previous three months. Class Ia antiarrhythmic drugs, such as disopyramide, quinidine and procainamide and other Class III drugs (e.g., amiodarone) are not recommended as concomitant therapy with sotalots (AF), because of their potential to prolong refractoriness. There is only limited experience with the concomitant use of Class Ib or Ic antiarrhythmics.
Congestive Heart Failure
Sympathetic stimulation is necessary in supporting circulatory function in congestive heart failure, and beta-blockade carries the potential hazard of further depressing myocardial contractility and precipitating more severe failure. In patients who have heart failure controlled by digitalis and/or diuretics, sotalol (AF) should be administered cautiously. Both digitalis and sotalol slow AV conduction. As with all beta-blockers, caution is advised when initiating therapy in patients with any evidence of left ventricular dysfunction. In a pooled data base of four placebo-controlled AFIB/AFL and PSVT studies, new or worsening CHF occurred during therapy with sotalol (AF) in 5 (1.2%) of 415 patients. In these studies patients with uncontrolled heart failure were excluded (i.e., NYHA Functional Classes III or IV). In other premarketing sotalol studies, new or worsened congestive heart failure (CHF) occurred in 3.3% (n=3257) of patients and led to discontinuation in approximately 1% of patients receiving sotalol. The incidence was higher in patients presenting with sustained ventricular tachycardia/ventricular fibrillation (4.6%, n=1363), or a prior history of heart failure (7.3%, n=696). Based on a life-table analysis, the one-year incidence of new or worsened CHF was 3% in patients without a prior history and 10% in patients with a prior history of CHF. NYHA Classification was also closely associated to the incidence of new or worsened heart failure while receiving sotalol (1.8% in 1395 Class I patients, 4.9% in 1254 Class II patients and 6.1% in 278 Class III or IV patients).
Electrolyte Disturbances
Sotalol (AF) should not be used in patients with hypokalemia or hypomagnesemia prior to correction of imbalance, as these conditions can exaggerate the degree of QT prolongation, and increase the potential for Torsade de Pointes. Special attention should be given to electrolyte and acid-base balance in patients experiencing severe or prolonged diarrhea or patients receiving concomitant diuretic drugs.
Bradycardia/Heart Block
The incidence of bradycardia (as determined by the investigators) in the supraventricular arrhythmia population treated with sotalol (AF) (N = 415) was 13%, and led to discontinuation in 2.4% of patients. Bradycardia itself increases the risk of Torsade de Pointes.
Recent Acute MI
Sotalol has been used in a controlled trial following an acute myocardial infarction without evidence of increased mortality (see Safety in Patients with Structural Heart Disease). Although specific studies of its use in treating atrial arrhythmias after infarction have not been conducted, the usual precautions regarding heart failure, avoidance of hypokalemia, bradycardia or prolonged QT interval apply.
Abrupt Withdrawal
Hypersensitivity to catecholamines has been observed in patients withdrawn from beta-blocker therapy. Occasional cases of exacerbation of angina pectoris, arrhythmias and, in some cases, myocardial infarction have been reported after abrupt discontinuation of beta-blocker therapy. Therefore, it is prudent when discontinuing chronically administered sotalol (AF), particularly in patients with ischemic heart disease, to carefully monitor the patient and consider the temporary use of an alternate beta-blocker if appropriate. If possible, the dosage of sotalol (AF) should be gradually reduced over a period of one to two weeks. If angina or acute coronary insufficiency develops, appropriate therapy should be instituted promptly. Patients should be warned against interruption or discontinuation of therapy without the physician's advice. Because coronary artery disease is common and may be unrecognized in patients receiving sotalols (AF), abrupt discontinuation in patients with arrhythmias may unmask latent coronary insufficiency.
Non-Allergic Bronchospasm (e.g., chronic bronchitis and emphysema)
PATIENTS WITH BRONCHOSPASTIC DISEASES SHOULD IN GENERAL NOT RECEIVE BETA-BLOCKERS. It is prudent, if sotalol hydrochloride (AF) is to be administered, to use the smallest effective dose, so that inhibition of bronchodilation produced by endogenous or exogenous catecholamine stimulation of beta2 receptors may be minimized.
Anaphylaxis
While taking beta-blockers, patients with a history of anaphylactic reaction to a variety of allergens may have a more severe reaction on repeated challenge, either accidental, diagnostic or therapeutic. Such patients may be unresponsive to the usual doses of epinephrine used to treat the allergic reaction.
Major Surgery
Chronically administered beta-blocking therapy should not be routinely withdrawn prior to major surgery; however, the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.
Diabetes
In patients with diabetes (especially labile diabetes) or with a history of episodes of spontaneous hypoglycemia, sotalol (AF) should be given with caution since beta-blockade may mask some important premonitory signs of acute hypoglycemia; e.g., tachycardia.
Sick Sinus Syndrome
Sotalol (AF) should be used only with extreme caution in patients with sick sinus syndrome associated with symptomatic arrhythmias, because it may cause sinus bradycardia, sinus pauses or sinus arrest. In patients with AFIB and sinus node dysfunction, the risk of Torsade de Pointes with sotalol (AF) therapy is increased, especially after cardioversion. Bradycardia following cardioversion in these patients is associated with QTc interval prolongation which is augmented due to the reverse use dependence of the Class III effects of sotalol (AF). Patients with AFIB/AFL associated with the sick sinus syndrome may be treated with sotalol (AF) if they have an implanted pacemaker for control of bradycardia symptoms.
Thyrotoxicosis
Beta-blockade may mask certain clinical signs (e.g., tachycardia) of hyperthyroidism. Patients suspected of developing thyrotoxicosis should be managed carefully to avoid abrupt withdrawal of beta-blockade which might be followed by an exacerbation of symptoms of hyperthyroidism, including thyroid storm. The beta-blocking effects of sotalol (AF) may be useful in controlling heart rate in AFIB associated with thyrotoxicosis but no study has been conducted to evaluate this. |clinicalTrials=Adverse events that are clearly related to sotalol AF are those which are typical of its Class II (beta-blocking) and Class III (cardiac action potential duration prolongation) effects. The common documented beta-blocking adverse events (bradycardia, dyspnea, and fatigue) and Class III effects (QT interval prolongation) are dose related.
In a pooled clinical trial population consisting of four placebo-controlled studies with 275 patients with AFIB/AFL treated with 160 to 320 mg doses of sotalol hydrochloride (AF), the following adverse events were reported at a rate of 2% or more in the 160-240 mg treated patients and greater than the rate in placebo patients (See Table 8). The data are presented by incidence of events in the sotalol (AF) and placebo groups by body system and daily dose. No significant irreversible non-cardiac end-organ toxicity was observed.

Overall, discontinuation because of unacceptable adverse events was necessary in 17% of the patients, and occurred in 10% of patients less than two weeks after starting treatment. The most common adverse events leading to discontinuation of sotalol hydrochloride (AF) were: fatigue 4.6%, bradycardia 2.4%, proarrhythmia 2.2%, dyspnea 2%, and QT interval prolongation 1.4%.
In clinical trials involving 1292 patients with sustained VT/VF, the common adverse events (occurring in ≥2% of patients) were similar to those described for the AFIB/AFL population.
Occasional reports of elevated serum liver enzymes have occurred with sotalol therapy but no cause and effect relationship has been established. One case of peripheral neuropathy which resolved on discontinuation of sotalol and recurred when the patient was rechallenged with the drug was reported in an early dose tolerance study. Elevated blood glucose levels and increased insulin requirements can occur in diabetic patients.
In an unblinded multicenter trial of 25 patients with SVT and/or VT receiving daily doses of 30, 90 and 210 mg/m2 with dosing every 8 hours for a total of 9 doses, no Torsades de Pointes or other serious new arrhythmias were observed. One (1) patient, receiving 30 mg/m2 daily, was discontinued because of increased frequency of sinus pauses/bradycardia. Additional cardiovascular AEs were seen at the 90 and 210 mg/m2 daily dose levels. They included QT prolongations (2 patients), sinus pauses/bradycardia (1 patient), increased severity of atrial flutter and reported chest pain (1 patient). Values for QTc ≥525 msec were seen in 2 patients at the 210 mg/m2 daily dose level. Serious adverse events including death, Torsades de Pointes, other proarrhythmias, high-degree AV blocks and bradycardia have been reported in infants and/or children.
Potential Adverse Effects
Foreign marketing experience with sotalol hydrochloride shows an adverse experience profile similar to that described above from clinical trials. Voluntary reports since introduction also include rare reports of: emotional liability, slightly clouded sensorium, incoordination, vertigo, paralysis, thrombocytopenia, eosinophilia, leukopenia, photosensitivity reaction, fever, pulmonary edema, hyperlipidemia, myalgia, pruritis, alopecia.
The oculomucocutaneous syndrome associated with the beta-blocker practolol has not been associated with sotalol (AF) during investigational use and foreign marketing experience.
|drugInteractions=* Drugs undergoing CYP450 metabolism: Sotalol is primarily eliminated by renal excretion; therefore, drugs that are metabolized by CYP450 are not expected to alter the pharmacokinetics of sotalol.
- Digoxin: Proarrhythmic events were more common in sotalol treated patients also receiving digoxin; it is not clear whether this represents an interaction or is related to the presence of CHF, a known risk factor for proarrhythmia, in the patients receiving digoxin. Both digitalis glycosides and beta-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia.
- Calcium blocking drugs: Sotalol (AF) should be administered with caution in conjunction with calcium blocking drugs because of possible additive effects on atrioventricular conduction or ventricular function. Additionally, concomitant use of these drugs may have additive effects on blood pressure, possibly leading to hypotension.
- Catecholamine-depleting agents: Concomitant use of catecholamine-depleting drugs, such as reserpine and guanethidine, with a beta-blocker may produce an excessive reduction of resting sympathetic nervous tone. Patients treated with sotalol (AF) plus a catecholamine depletor should therefore be closely monitored for evidence of hypotension and/or marked bradycardia which may produce syncope.
- Insulin and oral antidiabetics: Hyperglycemia may occur, and the dosage of insulin or antidiabetic drugs may require adjustment. Symptoms of hypoglycemia may be masked.
- Beta-2-receptor stimulants: Beta-agonists such as salbutamol, terbutaline and isoprenaline may have to be administered in increased dosages when used concomitantly with sotalol (AF).
- Clonidine: Beta-blocking drugs may potentiate the rebound hypertension sometimes observed after discontinuation of clonidine; therefore, caution is advised when discontinuing clonidine in patients receiving sotalol (AF).
- Other: No pharmacokinetic interactions were observed with hydrochlorothiazide or warfarin.
- Antacids: Administration of sotalol (AF) within 2 hours of antacids containing aluminum oxide and magnesium hydroxide should be avoided because it may result in a reduction in Cmax and AUC of 26% and 20%, respectively and consequently in a 25% reduction in the bradycardic effect at rest. Administration of the antacid two hours after sotalol (AF) has no effect on the pharmacokinetics or pharmacodynamics of sotalol.
- Drug/Laboratory Test Interactions: The presence of sotalol in the urine may result in falsely elevated levels of urinary metanephrine when measured by fluorimetric or photometric methods. In screening patients suspected of having a pheochromocytoma and being treated with sotalol, a specific method, such as a high performance liquid chromatographic assay with solid phase extraction (e.g., J. Chromatogr. 385:241, 1987) should be employed in determining levels of catecholamines.
|FDAPregCat=B |useInPregnancyFDA=Reproduction studies in rats and rabbits during organogenesis at 100 and 22 times the MRHD as mg/kg (9 and 7 times the MRHD as mg/m2), respectively, did not reveal any teratogenic potential associated with sotalol HCl. In rabbits, a high dose of sotalol HCl (160 mg/kg/day) at 16 times the MRHD as mg/kg (6 times the MRHD as mg/m2) produced a slight increase in fetal death likely due to maternal toxicity. Eight times the maximum dose (80 mg/kg/day or 3 times the MRHD as mg/m2) did not result in an increased incidence of fetal deaths. In rats, 1000 mg/kg/day sotalol HCl, 100 times the MRHD (18 times the MRHD as mg/m2), increased the number of early resorptions, while at 14 times the maximum dose (2.5 times the MRHD as mg/m2), no increase in early resorptions was noted. However, animal reproduction studies are not always predictive of human response.
Although there are no adequate and well-controlled studies in pregnant women, sotalol HCl has been shown to cross the placenta, and is found in amniotic fluid. There has been a report of subnormal birth weight with sotalol. Therefore, sotalol (AF) should be used during pregnancy only if the potential benefit outweighs the potential risk. |useInNursing=Sotalol is excreted in the milk of laboratory animals and has been reported to be present in human milk. Because of the potential for adverse reactions in nursing infants from sotalol (AF), a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. |useInPed=The safety and effectiveness of sotalol (AF) in children have not been established. However, the Class III electrophysiologic and beta-blocking effects, the pharmacokinetics, and the relationship between the effects (QTc interval and resting heart rate) and drug concentrations have been evaluated in children aged between 3 days and 12 years old. |useInRenalImpair=Sotalol hydrochloride (AF) is eliminated principally via the kidneys through glomerular filtration and to a small degree by tubular secretion. There is a direct relationship between renal function, as measured by serum creatinine or creatinine clearance, and the elimination rate of sotalol hydrochloride (AF). |administration=Oral/Intravenous |monitoring======Condition 1=====
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section) |IVCompat====Solution===
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Y-Site
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Admixture
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Syringe
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
TPN/TNA
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
|overdose====Acute Overdose===
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description) |drugBox={{Drugbox2 | verifiedrevid = 420038718 | IUPAC_name = (RS)-N-{4-[1-hydroxy-2-(propan-2-ylamino)ethyl]phenyl}methanesulfonamide | image = SotalolStructure.png | imagename = 1 : 1 mixture (racemate) | drug_name = Sotalol
| tradename = Betapace | Drugs.com = Monograph | MedlinePlus = a693010 | pregnancy_AU = | pregnancy_US = B | pregnancy_category = | legal_status = Rx-only | routes_of_administration = oral
| bioavailability = >95%
| metabolism = Not metabolized[citation needed]
| elimination_half-life = 12 hours
| excretion = Renal
Lactic (In lactating females)
| CASNo_Ref =
| CAS_number_Ref =
| CAS_number = 3930-20-9
| ATC_prefix = C07
| ATC_suffix = AA07
| PubChem = 5253
| DrugBank_Ref =
| DrugBank = DB00489
| ChemSpiderID_Ref =
| ChemSpiderID = 5063
| UNII_Ref =
| UNII = A6D97U294I
| KEGG_Ref =
| KEGG = D08525
| ChEMBL_Ref =
| ChEMBL = 471
| C=12 | H=20 | N=2 | O=3 | S=1
| molecular_weight = 272.3624 g/mol
| smiles = O=S(=O)(Nc1ccc(cc1)C(O)CNC(C)C)C
| InChI = 1/C12H20N2O3S/c1-9(2)13-8-12(15)10-4-6-11(7-5-10)14-18(3,16)17/h4-7,9,12-15H,8H2,1-3H3
| InChIKey = ZBMZVLHSJCTVON-UHFFFAOYAR
| StdInChI_Ref =
| StdInChI = 1S/C12H20N2O3S/c1-9(2)13-8-12(15)10-4-6-11(7-5-10)14-18(3,16)17/h4-7,9,12-15H,8H2,1-3H3
| StdInChIKey_Ref =
| StdInChIKey = ZBMZVLHSJCTVON-UHFFFAOYSA-N
}}
|mechAction=Sotalol hydrochloride has both beta-blocker (Vaughan Williams Class II) and cardiac action potential duration prolongation (Vaughan Williams Class III) antiarrhythmic properties. Sotalol hydrochloride is a racemic mixture of d- and l-sotalol. Both isomers have similar Class III antiarrhythmic effects, while the l-isomer is responsible for virtually all of the beta-blocking activity. The beta-blocking effect of sotalol is non-cardioselective, half maximal at about 80 mg/day and maximal at doses between 320 and 640 mg/day. Sotalol does not have partial agonist or membrane stabilizing activity. Although significant beta-blockade occurs at oral doses as low as 25 mg, significant Class III effects are seen only at daily doses of 160 mg and above.
In children, a Class III electrophysiologic effect can be seen at daily doses of 210 mg/m2 body surface area (BSA). A reduction of the resting heart rate due to the beta-blocking effect of sotalol is observed at daily doses ≥ 90 mg/m2 in children. |structure=Sotalol hydrochloride is an antiarrhythmic drug with Class II (beta-blocker) and Class III (cardiac action potential duration prolongation) properties. It is supplied as a light-blue, capsule-shaped tablet for oral administration. Sotalol hydrochloride is a white, crystalline solid with a molecular weight of 308.8. It is hydrophilic, soluble in water, propylene glycol and ethanol, but is only slightly soluble in chloroform. Chemically, sotalol hydrochloride is d,l-N -[4-[1-hydroxy-2-[(1-methylethyl) amino]ethyl]phenyl]methane-sulfonamide monohydrochloride. The molecular formula is C12H20N2O3 S·HCl and is represented by the following structural formula:

Satolol tablets contain the following inactive ingredients: microcrystalline cellulose, lactose, starch, stearic acid, magnesium stearate, colloidal silicon dioxide, and FD&C blue color #2 (aluminum lake, conc.). |PD=Sotalol hydrochloride prolongs the plateau phase of the cardiac action potential in the isolated myocyte, as well as in isolated tissue preparations of ventricular or atrial muscle (Class III activity). In intact animals it slows heart rate, decreases AV nodal conduction and increases the refractory periods of atrial and ventricular muscle and conduction tissue.
In man, the Class II (beta-blockade) electrophysiological effects of sotalol are manifested by increased sinus cycle length (slowed heart rate]), decreased AV nodal conduction and increased AV nodal refractoriness. The Class III electrophysiological effects in man include prolongation of the atrial and ventricular monophasic action potentials, and effective refractory period prolongation of atrial muscle, ventricular muscle, and atrio-ventricular accessory pathways (where present) in both the anterograde and retrograde directions. With oral doses of 160 to 640 mg/day, the surface ECG shows dose-related mean increases of 40-100 msec in QT and 10-40 msec in QTc. No significant alteration in QRS interval is observed.
In a small study (n=25) of patients with implanted defibrillators treated concurrently with sotalol, the average defibrillatory threshold was 6 joules (range 2-15 joules) compared to a mean of 16 joules for a nonrandomized comparative group primarily receiving amiodarone.
Twenty-five children in an unblinded, multicenter trial with supraventricular tachycardias (SVT) and/or ventricular tachyarrhythmias (VT), aged between 3 days and 12 years (mostly neonates and infants), received an ascending titration regimen with daily doses of 30, 90 and 210 mg/m2 with dosing every 8 hours for a total 9 doses. During steady-state, the respective average increases above baseline of the QTc interval, in msec (%), were 2(+1%), 14(+4%) and 29(+7%) msec at the 3 dose levels. The respective mean maximum increases above baseline of the QTc interval, in msec (%), were 23(+6%), 36(+9%) and 55(+14%) msec at the 3 dose levels. The steadystate percent increases in the RR interval were 3, 9 and 12%. The smallest children (BSA<0.33m2) showed a tendency for larger Class III effects (ΔQTc) and an increased frequency of prolongations of the QTc interval as compared with larger children (BSA≥0.33m2). The beta-blocking effects also tended to be greater in the smaller children (BSA<0.33m2). Both the Class III and beta-blocking effects of sotalol were linearly related with the plasma concentrations. |PK=In healthy subjects, the oral bioavailability of sotalol hydrochloride is 90-100%. After oral administration, peak plasma concentrations are reached in 2.5 to 4 hours, and steady-state plasma concentrations are attained within 2-3 days (i.e., after 5-6 doses when administered twice daily). Over the dosage range 160-640 mg/day sotalol hydrochloride displays dose proportionality with respect to plasma concentrations. Distribution occurs to a central (plasma) and to a peripheral compartment, with a mean elimination half-life of 12 hours. Dosing every 12 hours results in trough plasma concentrations which are approximately one-half of those at peak.
Sotalol hydrochloride does not bind to plasma proteins and is not metabolized. Sotalol hydrochloride shows very little intersubject variability in plasma levels. The pharmacokinetics of the d and l enantiomers of sotalol are essentially identical. Sotalol hydrochloride crosses the blood brain barrier poorly. Excretion is predominantly via the kidney in the unchanged form, and therefore lower doses are necessary in conditions of renal impairment. Age per se does not significantly alter the pharmacokinetics of sotalol, but impaired renal function in geriatric patients can increase the terminal elimination half-life, resulting in increased drug accumulation. The absorption of sotalol hydrochloride was reduced by approximately 20% compared to fasting when it was administered with a standard meal. Since sotalol hydrochloride is not subject to first-pass metabolism, patients with hepatic impairment show no alteration in clearance of sotalol.
The combined analysis of two unblinded, multicenter trials (a single dose and a multiple dose study) with 59 children, aged between 3 days and 12 years, showed the pharmacokinetics of sotalol to be first order. A daily dose of 30 mg/m2 of sotalol was administered in the single dose study and daily doses of 30, 90 and 210 mg/m2 were administered q 8h in the multi-dose study. After rapid absorption with peak levels occurring on average between 2-3 hours following administration, sotalol was eliminated with a mean half life of 9.5 hours. Steady-state was reached after 1-2 days. The average peak to trough concentration ratio was 2. BSA was the most important covariate and more relevant than age for the pharmacokinetics of sotalol.The smallest children (BSA<0.33m2) exhibited a greater drug exposure (+59%) than the larger children who showed a uniform drug concentration profile. The intersubject variation for oral clearance was 22%. |nonClinToxic=No evidence of carcinogenic potential was observed in rats during a 24-month study at 137-275 mg/kg/day (approximately 30 times the maximum recommended human oral dose (MRHD) as mg/kg or 5 times the MRHD as mg/m2) or in mice, during a 24-month study at 4141-7122 mg/kg/ day (approximately 450-750 times the MRHD as mg/kg or 36-63 times the MRHD as mg/m2).
Sotalol has not been evaluated in any specific assay of mutagenicity or clastogenicity. No significant reduction in fertility occurred in rats at oral doses of 1000 mg/kg/ day (approximately 100 times the MRHD as mg/kg or 9 times the MRHD as mg/m2) prior to mating, except for a small reduction in the number of offspring per litter. |clinicalStudies======Prolongation of Time to Recurrence of Symptomatic Atrial Fibrillation/ Flutter=====
Sotalol hydrochloride (AF) has been studied in patients with symptomatic AFIB/AFL in two principal studies, one in patients with primarily paroxysmal AFIB/AFL, the other in patients with primarily chronic AFIB.
In one study, a U.S. multicenter, randomized, placebo-controlled, double-blind, dose-response trial of patients with symptomatic primarily paroxysmal AFIB/AFL, three fixed dose levels of sotalol hydrochloride (AF) (80 mg, 120 mg and 160 mg) twice daily and placebo were compared in 253 patients. In patients with reduced creatinine clearance (40-60 mL/min) the same doses were given once daily. Patients were not randomized for the following reasons: QT >450 msec; creatinine clearance <40 mL/min; intolerance to beta-blockers; bradycardia-tachycardia syndrome in the absence of an implanted pacemaker; AFIB/AFL was asymptomatic or was associated with syncope, embolic CVA or TIA; acute myocardial infarction within the previous 2 months; congestive heart failure; bronchial asthma or other contraindications to beta-blocker therapy; receiving potassium losing diuretics without potassium replacement or without concurrent use of ACE-inhibitors; uncorrected hypokalemia (serum potassium <3.5 meq/L) or hypomagnesemia (serum magnesium <1.5 meq/L); received chronic oral amiodarone therapy for >1 month within previous 12 weeks; congenital or acquired long QT syndromes; history of Torsade de Pointes with other antiarrhythmic agents which increase the duration of ventricular repolarization; sinus rate <50 bpm during waking hours; unstable angina pectoris; receiving treatment with other drugs that prolong the QT interval; and AFIB/AFL associated with the Wolff-Parkinson-White syndrome (WPW). If the QT interval increased to ≥520 msec (or JT ≥430 msec if QRS >100 msec) the drug was discontinued. The patient population in this trial was 64% male, and the mean age was 62 years. No structural heart disease was present in 43% of the patients. Doses were administered once daily in 20% of the patients because of reduced creatinine clearance.
Sotalol hydrochloride (AF) was shown to prolong the time to the first symptomatic, ECG documented recurrence of AFIB/AFL, as well as to reduce the risk of such recurrence at both 6 and 12 months. The 120 mg dose was more effective than 80 mg, but 160 mg did not appear to have an added benefit. Note that these doses were given twice or once daily, depending on renal function. The results are shown in the figure and tables below.

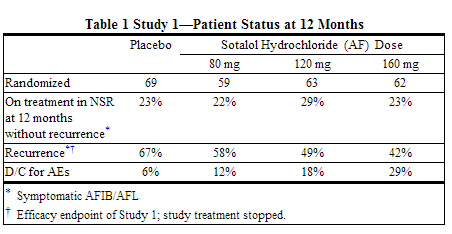
Please note that columns do not add up to 100% due to discontinuations (D/C) for "other" reasons.

Discontinuation because of adverse events was dose related.
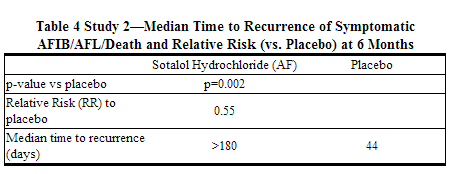
In a second multicenter, randomized, placebo-controlled, double-blind study of 6 months duration in 232 patients with chronic AFIB, sotalol hydrochloride (AF) was titrated over a dose range from 80 mg/day to 320 mg/day. The patient population of this trial was 70% male with a mean age of 65 years. Structural heart disease was present in 49% of the patients. All patients had chronic AFIB for >2 weeks but <1 year at entry with a mean duration of 4.1 months. Patients were excluded if they had significant electrolyte imbalance, QTc >460 msec, QRS >140 msec, any degree of AV block or functioning pacemaker, uncompensated cardiac failure, asthma, significant renal disease (estimated creatinine clearance <50 mL/min), heart rate <50 bpm, myocardial infarction or open heart surgery in past 2 months, unstable angina, infective endocarditis, active pericarditis or myocarditis, ≥ 3 DC cardioversions in the past, medications that prolonged QT interval, and previous amiodarone treatment. After successful cardioversion patients were randomized to receive placebo (n=114) or sotalol hydrochloride (AF) (n=118), at a starting dose of 80 mg twice daily. If the initial dose was not tolerated it was decreased to 80 mg once daily, but if it was tolerated it was increased to 160 mg twice daily. During the maintenance period 67% of treated patients received a dose of 160 mg twice daily, and the remainder received doses of 80 mg once daily (17%) and 80 mg twice daily (16%).
The figure and Tables below show the results of the trial. There was a longer time to ECG-documented recurrence of AFIB and a reduced risk of recurrence at 6 months compared to placebo.


Safety in Patients with Structural Heart Disease
In a multicenter double-blind randomized study reported by D. Julian et al, the effect of sotalol 320 mg once daily was compared with that of placebo in 1456 patients (randomized 3:2, sotalol to placebo) surviving an acute myocardial infarction (MI). Treatment was started 5 to 14 days after infarction. Patients were followed for 12 months. The mortality rate was 7.3% in the sotalol group and 8.9% in the placebo group, not a statistically significant difference. Although the results do not show evidence of a benefit of sotalol in this population, they do not show an added risk in post MI patients receiving sotalol. |howSupplied=(Description) |fdaPatientInfo=Prior to initiation of sotalol (AF) therapy, the patient should be advised to read the patient package insert and reread it each time therapy is renewed. The patient should be fully instructed on the need for compliance with the recommended dosing of sotalol (AF), the potential interactions with drugs that prolong the QT interval and other antiarrhythmics, and the need for periodic monitoring of QT and renal function to minimize the risk of serious abnormal rhythms.
Medications and Supplements
Assessment of patients' medication history should include all over-counter, prescription and herbal/natural preparations with emphasis on preparations that may affect the pharmacodynamics of sotalol (AF) such as other cardiac antiarrhythmic drugs, some phenothiazines, bepridil, tricyclic antidepressants and oral macrolides. Patients should be instructed to notify their health care providers of any change in over-the-counter, prescription or supplement use. If a patient is hospitalized or is prescribed a new medication for any condition, the patient must inform the health care provider of ongoing sotalol (AF) therapy. Patients should also check with their health care provider and/or pharmacist prior to taking a new over-the-counter medicine.
Electrolyte Imbalance
If patients experience symptoms that may be associated with electrolyte disturbances, such as excessive or prolonged diarrhea, sweating, or vomiting, or loss of appetite or thirst, these conditions should be immediately reported to their health care provider.
Dosing Schedule
Patients should be instructed NOT to double the next dose if a dose is missed. The next dose should be taken at the usual time.
|alcohol=Alcohol-Sotalol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
|lookAlike=* (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
|nlmPatientInfo=(Link to patient information page) |drugShortage=Drug Shortage }} } }