Sandbox:Usman Shah
Covid-19 Associated ARDS
Overview
Historical Perspective
- On 31 December 2019, the World Health Organization (WHO) was formally notified about a cluster of cases of pneumonia in Wuhan City.[1]
- Ten days later, WHO was aware of 282 confirmed cases, of which four were in Japan, South Korea and Thailand
- The virus responsible was isolated on 7 January and its genome shared on 12 January.The cause of the severe acute respiratory syndrome that became known as COVID‐19 was a novel coronavirus, SARS‐CoV‐2
- ARDS is one of the most important causes of hospital and ICU admission due to COVID.
- Many autopsies studies reported ARDS to be the cause of death in patients dying due to respiratory complications of COVID.
- As of July 19 2020 the number of total cases worldwide are 14,043,176 including 597,583 deaths, reported to WHO.
Classification
Authors in a case report highlighted the nonuniformity of patients with COVID-19-associated ARDS and proposed the existence of two primary phenotypes:
- Type L (low values of elastance, pulmonary ventilation/ perfusion ratio, lung weight, and recruitability).
- Type H (high values of elastance, right-to-left shunt, lung weight, and recruitability), more consistent with typical severe ARDS.[2]
ARDS is divided into three categories based on oxygenation index (PaO2/FiO2) on PEEP ≥ 5 cmH2O:
- mild (200 mmHg ≤ PaO2/FiO2 < 300 mmHg),
- mild-moderate (100 mmHg ≤ PaO2/FiO2 < 200 mmHg), and
- moderate-severe (PaO2/FiO2 < 100 mmHg).[3]
Pathophysiology
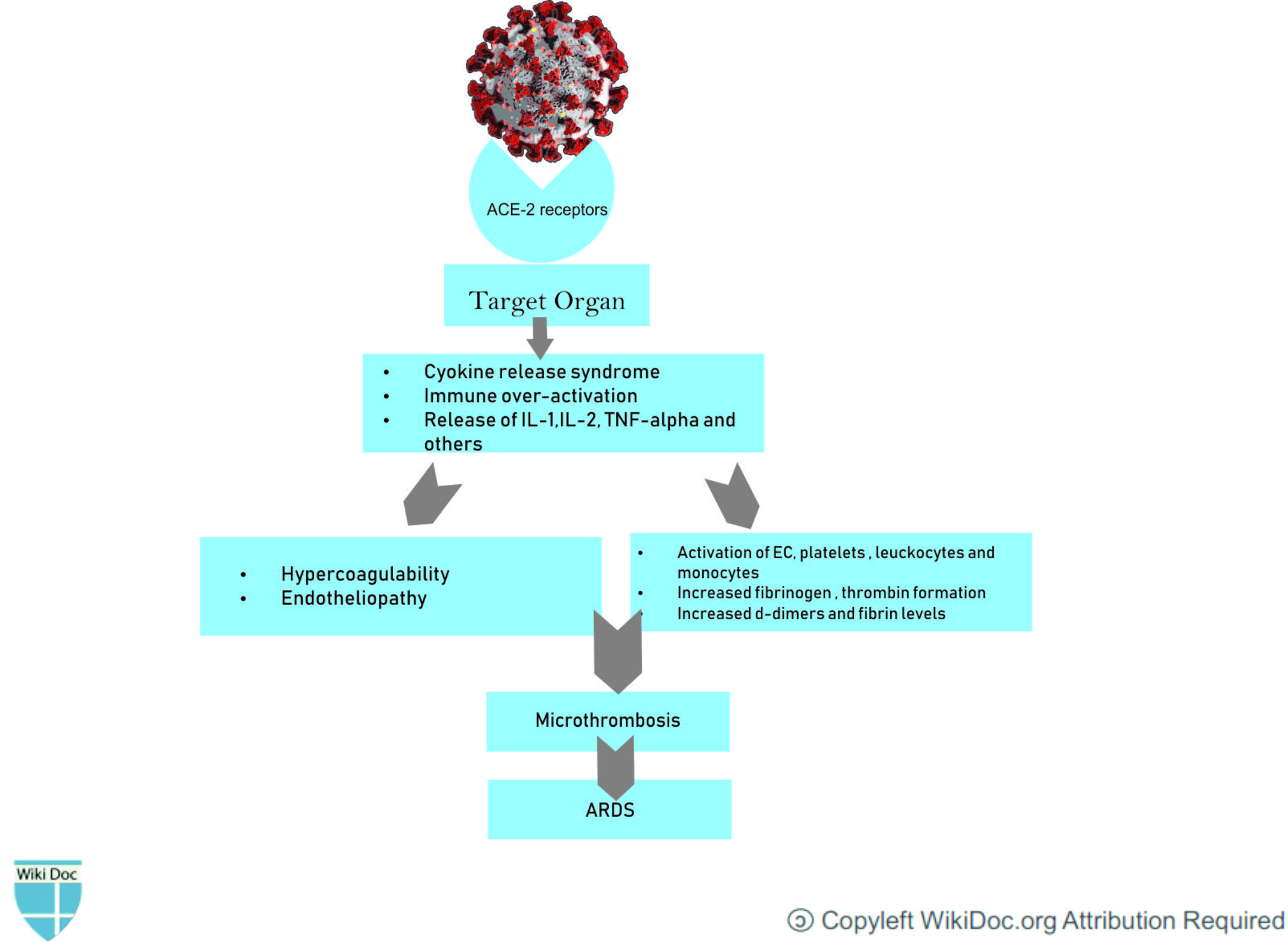
- The SARS-CoV-2 virus, like the closely-related MERS and SARS coronaviruses, effects its cellular entry via attachment of its virion spike protein (a.k.a. S protein) to the angiotensin-converting enzyme 2 (ACE2) receptor.[4]
- This receptor is commonly found on alveolar cells of the lung epithelium.It suggested that injury to the alveolar epithelial cells was the main cause of COVID-19-related ARDS.
- Cellular infection and viral replication cause activation of the inflammasome in the host cell, leading to the release of pro-inflammatory cytokines and cell death by pyroptosis with ensuing release of a damage-associated molecular pattern, further amplifying the inflammatory response.[5]
- The cytokine storm and the deadly uncontrolled systemic inflammatory response resulting from the release of large amounts of proinflammatory cytokines including interferons and interleukins and, chemokines by immune effector cells resulting in acute inflammation within the alveolar space. The exudate containing plasma proteins, including albumin, fibrinogen, proinflammatory cytokines and coagulation factors will increase alveolar-capillary permeability and decrease the normal gas exchange and plasma proteins, including albumin, fibrinogen, proinflammatory cytokines and coagulation factors.[6]
- In line with this, recent studies have shown that patients with COVID-19 have high levels of inflammatory cytokines, such as interleukin (IL)-1β, IL-2, IL-6 IL-7, IL-8, IL-9, IL-10, IL-18, tumor necrosis factor (TNF)-α, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor, fibroblast growth factor, macrophage inflammatory protein 1, compared to healthy individuals.
- Circulating levels of IL-6, IL-10, and TNF-α also correlated with illness severity as they were significantly higher in intensive care unit (ICU) patients compared to mild/moderate cases. In particular, IL-6 may suppress normal T-cell activation and TNF-α can promote T-cell apoptosis via interacting with its receptor TNF receptor 1, and their upregulation may in part contribute to lymphocytopenia, a feature often encountered in COVID-19, with a more pronounced decline in severe cases [5]
- IL-6 is not the only protagonist on the scene. It was proved, for instance, that the binding of SARS-CoV-2 to the Toll-Like Receptor (TLR) induces the release of pro-IL-1β which is cleaved into the active mature IL-1β mediating lung inflammation, until fibrosis.[7]
- This inflammatory process leads to the fibrin deposition in the air spaces and lung parenchyma and contributes to hyaline-membrane formation and subsequent alveolar fibrosis.[8]
- Patients infected with COVID‐19 also exhibit coagulation abnormalities.This procoagulant pattern can lead to acute respiratory distress syndrome[9]
Differentiating COVID-associated ARDS from other Diseases
- Large observational studies suggest that patients with COVID-19-associated ARDS have similar respiratory system mechanics to patients with ARDS from other causes and that, for most patients, COVID-19-associated ARDS is, in the end, ARDS. However, lung compliance might be relatively normal in some COVID-19-related ARDS patients who met ARDS Berlin criteria. This was obviously inconsistent with ARDS caused by other factors. In addition, the lung compliance was relatively high in some COVID-19-related ARDS patients, which was inconsistent with the severity of hypoxemia.[2]
- COVID-19 associated ARDS can be differentiated from H1N1 another very common cause ARDS caused by a viral infection
- Compared with H1N1 patients, patients with COVID-19-induced ARDS had lower severity of illness scores at presentation and lower SOFA score-adjusted mortality.Ground-glass opacities was more common in patients with COVID-19 than in patients with H1N1 [10]
- Pulmonary thrombosis is also associated with COVID-19 related ARDS.
- And most importantly Positive SARS-CoV-2 infection on PCR.
Epidemiology and Demographics
Incidence of ARDS in Covid Patients
- A meta-analysis which included 50,466 COVID-19 cases described an ARDS incidence of 14.8% (95% CI: 4.6-29.6).[11]
Age
- Covid-19 affects all age groups
- In a retrospective cohort study of 201 hospitalised patients with confirmed COVID-19 pneumonia, 84 (41.8%) developed ARDS. The median age of ARDS patients was 58.5 years, compared with 48 years for non-ARDS patients. They calculated being aged 65 years or over was associated with a 3.26 increased risk of ARDS (95% CI 2.08-5.11 p<0.001) compared to the under 65s. They also found that patients who developed ARDS and were aged 65 years or over had a 6.17 increased risk of death (95% CI, 3.26-11.67; P<0.001) compared to ARDS patients under 65.[11]
Gender
- Some case studies report that men are more commonly affected by ARDS than women.
- In the public data set, the number of men who died from COVID-19 is 2.4 times that of women (70.3 vs. 29.7%, P = 0.016).
Race
- A large study in the United States reported that that African Americans were at a higher risk of ARDS than white individuals.\
Risk Factors
Increase Risk of developing ARDS in relation to Comorbidities
- Older age (≥65 years old)
- High fever (≥39 °C)
- Comorbidities (eg, hypertension, diabetes)
- Neutrophilia
- Lymphocytopenia (as well as lower CD3 and CD4 T-cell counts)
- Elevated end-organ related indices (eg, AST, urea, LDH)
- Elevated inflammation-related indices (high-sensitivity C-reactive protein and serum ferritin)
- Elevated coagulation function–related indicators (PT and D-dimer)
Reported hazard ratio for risk developing ARDS in relation to selected laboratory results
| Hazard Ratio | 95% CI interval | P-value | |
|---|---|---|---|
| Higher LDH | 1.40 | 1.44-1.79 | <0.001 |
| Higher D-Dimer | 1.03 | 1.01-1.04 | <0.001 |
| Higher Neutrophils | 1.14 | 1.09-119 | <0.001 |
Natural History, Complications and Prognosis
The natural history of ARDS is hallmarked by three histopathological phases—exudative, proliferative, and fibrotic phase—each correlated to distinctive clinical manifestations.
Exudative Phase
- he exudative phase typically encompasses the first 5 to 7 days of illness after exposure to one or more precipitation factors.
- Histopathologically, loss of integrity of the alveolar barrier results in the influx of proteinaceous fluid into the air place, and formation of the hyaline membrane. Pulmonary edema and atelectasis with reduced pulmonary compliance ensue, leading to the development of pulmonary shunt and hypoxemia.
- In this phase, patients experience respiratory symptoms including dyspnea, tachypnea, and increased work of breathing that eventually result in respiratory failure requiring ventilator support. If left untreated, approximately 70% of patients with ARDS may progress to mortality.Among non-survivors, approximately 50% patients die within a week of the onset with exudative change as the predominant histopathological feature
Proliferative Phase
- The proliferative phase generally lasts from day 7 to day 21.
- Histopathologically, reparative processes take place in the injured alveoli, including organization of exudates, a shift to lymphocyte-predominant infiltrates, and proliferation of type II pneumocytes.
- In this phase, patients may recover from acute respiratory distress despite the persistence of residual symptoms. Patients who do not recover during this phase develop progressive lung injury and early changes of fibrosis.
Fibrotic Phase
- The fibrotic phase occurs 3 to 4 weeks following the initial pulmonary insult.
- Histopathologically, extensive fibrosis is prominent in the alveolar interstitium and duct, with disruption of acinar architecture and emphysema-like changes.
- The evidence for pulmonary fibrosis on biopsy is associated with increased mortality.
Complications
Complications may include the following:
- Lungs: barotrauma (volutrauma), pulmonary embolism (PE), pulmonary fibrosis, ventilator-associated pneumonia (VAP)
- Gastrointestinal: bleeding (ulcer), dysmotility, pneumoperitoneum, bacterial translocation
- Neurological: hypoxic brain damage
- Cardiac: abnormal heart rhythms, myocardial dysfunction
- Kidney: acute kidney failure, positive fluid balance
- Mechanical: vascular injury, pneumothorax (by placing pulmonary artery catheter), tracheal injury/stenosis (result of intubation and/or irritation by endotracheal tube)
- Nutritional: malnutrition (catabolic state), electrolyte abnormalities Other complications that are typically associated with ARDS include:
- Atelectasis: small air pockets within the lung collapse
- Complications that arise from treatment in a hospital: blood clots formed by lying down for long periods of time, weakness in muscles that are used for breathing, stress ulcers, and even depression or other mental illnesses.
- Failure of multiple organs
- Pulmonary hypertension or increase in blood pressure in the main artery from the heart to the lungs. This complication typically occurs due to the restriction of the blood vessel due to inflammation of the mechanical ventilation
Prognosis
- The overall prognosis of ARDS is poor, with mortality rates of approximately 40%. Exercise limitation, physical and psychological sequelae, decreased physical quality of life, and increased costs and use of health care services are important sequelae of ARDS.
Diagnosis
COVID-19 ARDS is diagnosed when someone with confirmed COVID-19 infection meets the Berlin 2012 ARDS diagnostic criteria of: [12]
- acute hypoxemic respiratory failure,
- presentation within 1 week of worsening respiratory symptoms;
- bilateral airspace disease on chest x-ray, computed tomography, or ultrasound that is not fully explained by effusions, lobar or lung collapse, or nodules;
- and cardiac failure is not the primary cause of acute hypoxemic respiratory failure.
 |
| Criteria | Symptomatic WM | Asymptomatic WM | IgM-Related Disorders | MGUS |
|---|---|---|---|---|
| IgM monoclonal protein | + | + | + | + |
| Bone marrow infiltration | + | + | - | - |
| Symptoms attributable to IgM | + | - | + | - |
| Symptoms attributable to tumor infiltration | + | - | - | - |
|
Infra-Hisian Block Microchapters |
References
- ↑ 1.0 1.1 Chaplin, Steve (2020). "COVID
‐19: a brief history and treatments in development". Prescriber. 31 (5): 23–28. doi:10.1002/psb.1843. ISSN 0959-6682. line feed character in
|title=at position 6 (help) - ↑ 2.0 2.1 Fan, Eddy; Beitler, Jeremy R; Brochard, Laurent; Calfee, Carolyn S; Ferguson, Niall D; Slutsky, Arthur S; Brodie, Daniel (2020). "COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted?". The Lancet Respiratory Medicine. doi:10.1016/S2213-2600(20)30304-0. ISSN 2213-2600.
- ↑ Li, Xu; Ma, Xiaochun (2020). "Acute respiratory failure in COVID-19: is it "typical" ARDS?". Critical Care. 24 (1). doi:10.1186/s13054-020-02911-9. ISSN 1364-8535.
- ↑ "COVID-19 | Radiology Reference Article | Radiopaedia.org".
- ↑ 5.0 5.1 Iannaccone, Giulia; Scacciavillani, Roberto; Del Buono, Marco Giuseppe; Camilli, Massimiliano; Ronco, Claudio; Lavie, Carl J.; Abbate, Antonio; Crea, Filippo; Massetti, Massimo; Aspromonte, Nadia (2020). "Weathering the Cytokine Storm in COVID-19: Therapeutic Implications". Cardiorenal Medicine: 1–11. doi:10.1159/000509483. ISSN 1664-3828.
- ↑ Meduri, G. Umberto; Annane, Djillali; Chrousos, George P.; Marik, Paul E.; Sinclair, Scott E. (2009). "Activation and Regulation of Systemic Inflammation in ARDS". Chest. 136 (6): 1631–1643. doi:10.1378/chest.08-2408. ISSN 0012-3692.
- ↑ Bertozzi, Paul; Astedt, Birgir; Zenzius, Laura; Lynch, Karen; LeMaire, Françoise; Zapol, Warren; Chapman, Harold A. (1990). "Depressed Bronchoalveolar Urokinase Activity in Patients with Adult Respiratory Distress Syndrome". New England Journal of Medicine. 322 (13): 890–897. doi:10.1056/NEJM199003293221304. ISSN 0028-4793.
- ↑ Ranucci, Marco; Ballotta, Andrea; Di Dedda, Umberto; Bayshnikova, Ekaterina; Dei Poli, Marco; Resta, Marco; Falco, Mara; Albano, Giovanni; Menicanti, Lorenzo (2020). "The procoagulant pattern of patients with COVID‐19 acute respiratory distress syndrome". Journal of Thrombosis and Haemostasis. 18 (7): 1747–1751. doi:10.1111/jth.14854. ISSN 1538-7933.
- ↑ Tang X, Du RH, Wang R, Cao TZ, Guan LL, Yang CQ, Zhu Q, Hu M, Li XY, Li Y, Liang LR, Tong ZH, Sun B, Peng P, Shi HZ (July 2020). "Comparison of Hospitalized Patients With ARDS Caused by COVID-19 and H1N1". Chest. 158 (1): 195–205. doi:10.1016/j.chest.2020.03.032. PMID 32224074 Check
|pmid=value (help). - ↑ 11.0 11.1 "Are there risk factors and preventative interventions for acute respiratory distress syndrome (ARDS) in COVID-19? - CEBM".
- ↑ "COVID-19 ARDS: clinical features and differences to "usual" pre-COVID ARDS | The Medical Journal of Australia".
- ↑ Kiran U, Aggarwal S, Choudhary A, Uma B, Kapoor PM (2017). "The blalock and taussig shunt revisited". Ann Card Anaesth. 20 (3): 323–330. doi:10.4103/aca.ACA_80_17. PMC 5535574. PMID 28701598.