Prostate cancer medical therapy: Difference between revisions
| Line 29: | Line 29: | ||
Side effects of radiation therapy might occur after a few weeks into treatment. Both types of radiation therapy may cause [[diarrhea]] and mild [[Gastrointestinal bleeding|rectal bleeding]] due to [[radiation proctitis]], as well as urinary incontinence and impotence. Symptoms tend to improve over time. Rates for impotence when comparing radiation to nerve-sparing surgery are similar. Radiation has lower rates of incontinence but higher rates of occasional mild rectal bleeding.<ref>{{cite journal| last=Lawton| first=CA| coauthors=Won M, Pilepich MV, Asbell SO, Shipley WU, Hanks GE, Cox JD, Perez CA, Sause WT, Doggett SR, et al| title=Long-term treatment sequelae following external beam irradiation for adenocarcinoma of the prostate: analysis of RTOG studies 7506 and 7706| journal=Int J Radiat Oncol Biol Phys| year=1991| month=September| volume=21| issue=4| pages=935–9| pmid=1917622}}</ref> Men who have undergone external beam radiation therapy may have a slightly higher risk of later developing [[colon cancer]] and [[bladder cancer]].<ref>{{cite journal| last=Brenner| first=DJ| coauthors=Curtis RE, Hall EJ, Ron E| title=Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery| journal=Cancer| year=2000| month=January 15| volume=88| issue=2| pages=398–406| pmid=10640974| doi=10.1002/(SICI)1097-0142(20000115)88:2<398::AID-CNCR22>3.0.CO;2-V}}</ref> | Side effects of radiation therapy might occur after a few weeks into treatment. Both types of radiation therapy may cause [[diarrhea]] and mild [[Gastrointestinal bleeding|rectal bleeding]] due to [[radiation proctitis]], as well as urinary incontinence and impotence. Symptoms tend to improve over time. Rates for impotence when comparing radiation to nerve-sparing surgery are similar. Radiation has lower rates of incontinence but higher rates of occasional mild rectal bleeding.<ref>{{cite journal| last=Lawton| first=CA| coauthors=Won M, Pilepich MV, Asbell SO, Shipley WU, Hanks GE, Cox JD, Perez CA, Sause WT, Doggett SR, et al| title=Long-term treatment sequelae following external beam irradiation for adenocarcinoma of the prostate: analysis of RTOG studies 7506 and 7706| journal=Int J Radiat Oncol Biol Phys| year=1991| month=September| volume=21| issue=4| pages=935–9| pmid=1917622}}</ref> Men who have undergone external beam radiation therapy may have a slightly higher risk of later developing [[colon cancer]] and [[bladder cancer]].<ref>{{cite journal| last=Brenner| first=DJ| coauthors=Curtis RE, Hall EJ, Ron E| title=Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery| journal=Cancer| year=2000| month=January 15| volume=88| issue=2| pages=398–406| pmid=10640974| doi=10.1002/(SICI)1097-0142(20000115)88:2<398::AID-CNCR22>3.0.CO;2-V}}</ref> | ||
===Hormonal therapy=== | |||
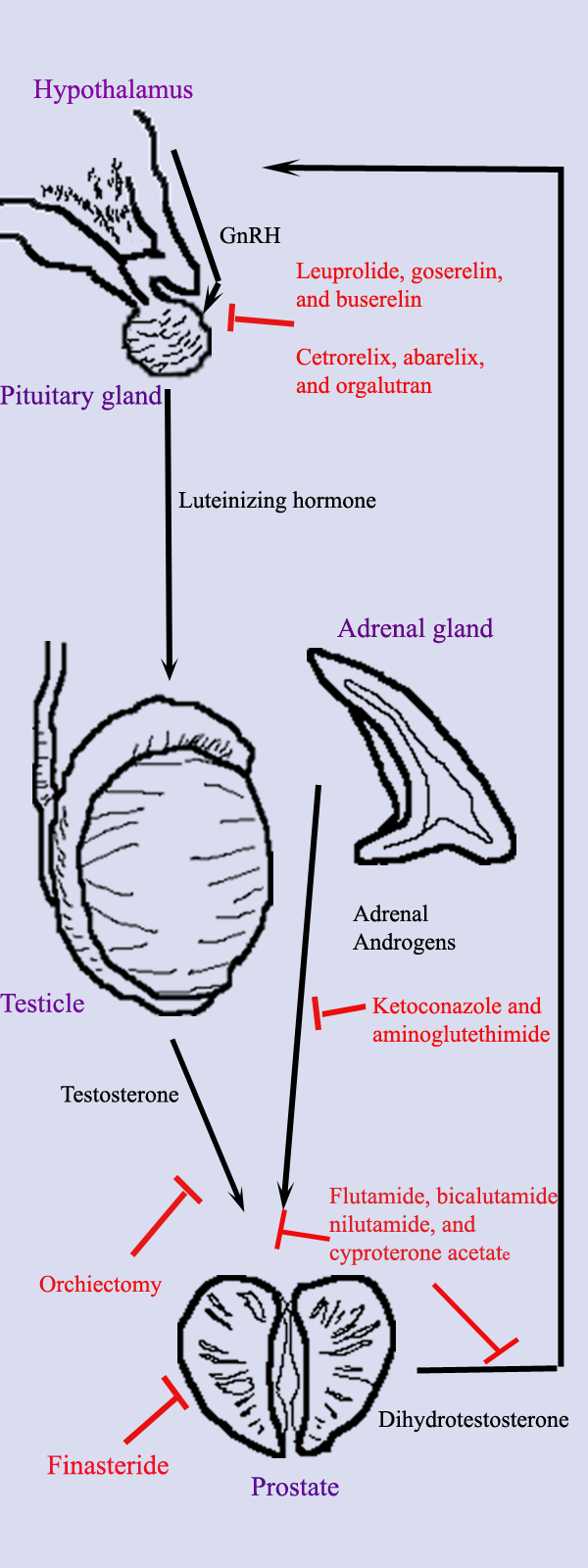
[[Image:prostatehormone.jpg|right|thumb|200px|'''Hormonal therapy in prostate cancer.''' Diagram shows the different organs (''purple text''), hormones (''black text and arrows''), and treatments (''red text and arrows'') important in hormonal therapy.]] | |||
[[Hormonal therapy (oncology)|Hormonal therapy]] uses medications or surgery to block prostate cancer cells from getting [[dihydrotestosterone]] (DHT), a hormone produced in the prostate and required for the growth and spread of most prostate cancer cells. Blocking DHT often causes prostate cancer to stop growing and even shrink. However, hormonal therapy rarely cures prostate cancer because cancers which initially respond to hormonal therapy typically become resistant after one to two years. Hormonal therapy is therefore usually used when cancer has spread from the prostate. It may also be given to certain men undergoing radiation therapy or surgery to help prevent return of their cancer.<ref>{{cite journal| last=Robson| first=M| coauthors=Dawson N| title=How is androgen-dependent metastatic prostate cancer best treated?| journal=Hematol Oncol Clin North Am| year=1996| month=June| volume=10| issue=3| pages=727–47| pmid=8773508| doi=10.1016/S0889-8588(05)70364-6}} Review.</ref> | |||
Hormonal therapy for prostate cancer targets the pathways the body uses to produce DHT. A [[feedback loop]] involving the testicles, the hypothalamus, and the pituitary, adrenal, and prostate glands controls the blood levels of DHT. First, low blood levels of DHT stimulate the [[hypothalamus]] to produce [[gonadotropin releasing hormone]] (GnRH). GnRH then stimulates the [[pituitary gland]] to produce [[luteinizing hormone]] (LH), and LH stimulates the [[testicles]] to produce testosterone. Finally, testosterone from the testicles and dehydroepiandrosterone from the [[adrenal gland]]s stimulate the prostate to produce more DHT. Hormonal therapy can decrease levels of DHT by interrupting this pathway at any point. | |||
There are several forms of hormonal therapy: | |||
*[[Castration|Orchiectomy]] is surgery to remove the testicles. Because the testicles make most of the body's testosterone, after orchiectomy testosterone levels drop. Now the prostate not only lacks the testosterone stimulus to produce DHT, but also it does not have enough testosterone to transform into DHT. | |||
*[[Antiandrogens]] are medications such as [[flutamide]], [[bicalutamide]], [[nilutamide]], and [[cyproterone acetate]] which directly block the actions of testosterone and DHT within prostate cancer cells. | |||
*Medications which block the production of adrenal androgens such as DHEA include [[ketoconazole]] and [[aminoglutethimide]]. Because the adrenal glands only make about 5% of the body's androgens, these medications are generally used only in combination with other methods that can block the 95% of androgens made by the testicles. These combined methods are called total androgen blockade (TAB). TAB can also be achieved using antiandrogens. | |||
*GnRH action can be interrupted in one of two ways. [[Gonadotropin-releasing hormone analog|GnRH antagonists]] suppress the production of LH directly, while [[Gonadotropin-releasing hormone analog|GnRH agonists]] suppress LH through the process of [[downregulation]] after an initial stimulation effect. [[Abarelix]] is an example of a GnRH antagonist, while the GnRH agonists include [[leuprolide]], [[goserelin]], [[triptorelin]], and [[buserelin]]. Initially, GnRH agonists ''increase'' the production of LH. However, because the constant supply of the medication does not match the body's natural production rhythm, production of both LH and GnRH decreases after a few weeks.<ref>{{cite journal| last=Loblaw| first=DA| coauthors=Mendelson DS, Talcott JA, Virgo KS, Somerfield MR, Ben-Josef E, Middleton R, Porterfield H, Sharp SA, Smith TJ, Taplin ME, Vogelzang NJ, Wade JL Jr, Bennett CL, Scher HI; American Society of Clinical Oncology| title=American Society of Clinical Oncology recommendations for the initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer| journal=J Clin Oncol| year=2004| month=July 15| volume=22| issue=14| pages=2927–41| pmid=15184404| doi=10.1200/JCO.2004.04.579}} Erratum in: J Clin Oncol. 2004 November 1;22(21):4435.</ref> | |||
*A very recent Trial I study (N=21) found that [[Abiraterone|Abiraterone Acetate]] caused dramatic reduction in [[PSA]] levels and [[Tumor]] sizes in aggressive end-stage prostate cancer for 70% of patients. This is prostate cancer that resists all other treatments (e.g., castration, other hormones, etc.). Officially the impacts on life-span are not yet known because subjects have not been taking the drug very long. Larger Trial III Clinical Studies are in the works. If successful an approved treatment is hoped for around 2011.<ref>{{cite journal| last=de Bono| first=Johann| coauthors= Gerhardt Attard, Alison H.M. Reid, Timothy A. Yap, Florence Raynaud, Mitch Dowsett, Sarah Settatree, Mary Barrett, Christopher Parker, Vanessa Martins, Elizabeth Folkerd, Jeremy Clark, Colin S. Cooper, Stan B. Kaye, David Dearnaley, Gloria Lee | title= Phase I Clinical Trial of a Selective Inhibitor of CYP17, Abiraterone Acetate, Confirms That Castration-Resistant Prostate Cancer Commonly Remains Hormone Driven| url = http://jco.ascopubs.org/cgi/content/abstract/JCO.2007.15.9749v1 | journal=J Clin Oncol| year=2004| month=July 21| volume= | issue= | pages= online| pmid=15184404| doi=10.1200/JCO.2007.15.9749| nopp=true}} Erratum in: J Clin Oncol. Early Release, published ahead of print July 21, 2008</ref><ref>{{ cite news | author = Richard Warry | title = Drug for deadly prostate cancer | url = http://news.bbc.co.uk/2/hi/health/7517414.stm | publisher = [[BBC]] | date = July 22, 2008 | accessdate = 2008-07-23 }}</ref> | |||
The most successful hormonal treatments are orchiectomy and GnRH agonists. Despite their higher cost, GnRH agonists are often chosen over orchiectomy for cosmetic and emotional reasons. Eventually, total androgen blockade may prove to be better than orchiectomy or GnRH agonists used alone. | |||
Each treatment has disadvantages which limit its use in certain circumstances. Although orchiectomy is a low-risk surgery, the psychological impact of removing the testicles can be significant. The loss of testosterone also causes [[Hot flush|hot flashes]], weight gain, loss of [[libido]], enlargement of the [[breast]]s ([[gynecomastia]]), impotence and [[osteoporosis]]. GnRH agonists eventually cause the same side effects as orchiectomy but may cause worse symptoms at the beginning of treatment. When GnRH agonists are first used, testosterone surges can lead to increased bone pain from metastatic cancer, so antiandrogens or abarelix are often added to blunt these side effects. Estrogens are not commonly used because they increase the risk for [[cardiovascular disease]] and [[thrombosis|blood clots]]. The antiandrogens do not generally cause impotence and usually cause less loss of bone and muscle mass. Ketoconazole can cause [[Hepatotoxicity|liver damage]] with prolonged use, and aminoglutethimide can cause skin [[rash]]es. | |||
==References== | ==References== | ||
{{Reflist|2}} | {{Reflist|2}} | ||
Revision as of 17:27, 15 December 2011
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
|
Prostate cancer Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Prostate cancer medical therapy On the Web |
|
American Roentgen Ray Society Images of Prostate cancer medical therapy |
|
Risk calculators and risk factors for Prostate cancer medical therapy |
Overview
Therapy
Natural therapy
As an alternative to active surveillance or invasive treatments, which does nothing to change the course of disease, a growing number of clinicians and researchers are looking at non-invasive ways to help men with apparently localized prostate cancer. Perhaps most convincing among this group are Dean Ornish, MD and colleagues, previously made famous for showing that aggressive lifestyle changes can reverse atherosclerosis, and now showing that PSA can be lowered in men with apparent localized prostate cancer using a vegan diet (fish allowed), regular exercise, and stress reduction.[1] These results have so far proven durable after two-years' treatment.[2]
Many other single agents have been shown to reduce PSA, slow PSA doubling times, or have similar effects on secondary markers in men with localized cancer in short term trials, such as the Wonderful variety of pomegranate juice 8 oz daily or genistein, an isoflavone found in various legumes, 60 mg per day.[3][4] The potential of using multiple such agents in concert, let alone combining them with lifestyle changes, has not yet been studied but the potential is great. This is particularly true because most of these natural approaches have very low adverse effect rates, and in fact tend to help other risk factors and disease conditions such as atherosclerosis, diabetes, and risk for other cancers at the same time they are helping slow down prostate cancer. A more thorough review of natural approaches to prostate cancer has been published.[5]
Radiation therapy
Radiation therapy, also known as radiotherapy, is often used to treat all stages of prostate cancer, or when surgery fails. Radiotherapy uses ionizing radiation to kill prostate cancer cells. When absorbed in tissue, Ionizing radiation such as Gamma and x-rays damage the DNA in cells, which increases the probability of apoptosis (cell death). Two different kinds of radiation therapy are used in prostate cancer treatment: external beam radiation therapy and brachytherapy (specifically prostate brachytherapy).
External beam radiation therapy uses a linear accelerator to produce high-energy x-rays which are directed in a beam towards the prostate. A technique called Intensity Modulated Radiation Therapy (IMRT) may be used to adjust the radiation beam to conform with the shape of the tumor, allowing higher doses to be given to the prostate and seminal vesicles with less damage to the bladder and rectum. External beam radiation therapy is generally given over several weeks, with daily visits to a radiation therapy center. New types of radiation therapy may have fewer side effects than traditional treatment. One of these is Tomotherapy.
Permanent implant brachytherapy is a popular treatment choice for patients with low to intermediate risk features, can be performed on an outpatient basis, and is associated with good 10-year outcomes with relatively low morbidity[6] It involves the placement of about 100 small "seeds" containing radioactive material (such as iodine-125 or palladium-103) with a needle through the skin of the perineum directly into the tumor while under spinal or general anesthetic. These seeds emit lower-energy X-rays which are only able to travel a short distance. Although the seeds eventually become inert, they remain in the prostate permanently. The risk of exposure to others from men with implanted seeds is generally accepted to be insignificant.[7]
Radiation therapy is commonly used in prostate cancer treatment. It may be used instead of surgery or after surgery in early stage prostate cancer. In advanced stages of prostate cancer radiation is used to treat painful bone metastases. Radiation treatments also can be combined with hormonal therapy for intermediate risk disease, when radiation therapy alone is less likely to cure the cancer. Some radiation oncologists combine external beam radiation and brachytherapy for intermediate to high risk situations. One study found that the combination of six months of androgen suppressive therapy combined with external beam radiation had improved survival compared to radiation alone in patients with localized prostate cancer.[8] Others use a "triple modality" combination of external beam radiation therapy, brachytherapy, and hormonal therapy.
Radiation therapy uses high-energy rays or particles to kill cancer cells.[9] When delivered in the correct dosage, radiation can reduce the risk of recurrence.
Less common applications for radiotherapy are when cancer is compressing the spinal cord, or sometimes after surgery, such as when cancer is found in the seminal vesicles, in the lymph nodes, outside the prostate capsule, or at the margins of the biopsy.
Radiation therapy is often offered to men whose medical problems make surgery more risky. Radiation therapy appears to cure small tumors that are confined to the prostate just about as well as surgery. However, some issues remain unresolved, such as whether radiation should be given to the rest of the pelvis, how much the absorbed dose should be, and whether hormonal therapy should be given at the same time.
Side effects of radiation therapy might occur after a few weeks into treatment. Both types of radiation therapy may cause diarrhea and mild rectal bleeding due to radiation proctitis, as well as urinary incontinence and impotence. Symptoms tend to improve over time. Rates for impotence when comparing radiation to nerve-sparing surgery are similar. Radiation has lower rates of incontinence but higher rates of occasional mild rectal bleeding.[10] Men who have undergone external beam radiation therapy may have a slightly higher risk of later developing colon cancer and bladder cancer.[11]
Hormonal therapy
Hormonal therapy uses medications or surgery to block prostate cancer cells from getting dihydrotestosterone (DHT), a hormone produced in the prostate and required for the growth and spread of most prostate cancer cells. Blocking DHT often causes prostate cancer to stop growing and even shrink. However, hormonal therapy rarely cures prostate cancer because cancers which initially respond to hormonal therapy typically become resistant after one to two years. Hormonal therapy is therefore usually used when cancer has spread from the prostate. It may also be given to certain men undergoing radiation therapy or surgery to help prevent return of their cancer.[12]
Hormonal therapy for prostate cancer targets the pathways the body uses to produce DHT. A feedback loop involving the testicles, the hypothalamus, and the pituitary, adrenal, and prostate glands controls the blood levels of DHT. First, low blood levels of DHT stimulate the hypothalamus to produce gonadotropin releasing hormone (GnRH). GnRH then stimulates the pituitary gland to produce luteinizing hormone (LH), and LH stimulates the testicles to produce testosterone. Finally, testosterone from the testicles and dehydroepiandrosterone from the adrenal glands stimulate the prostate to produce more DHT. Hormonal therapy can decrease levels of DHT by interrupting this pathway at any point. There are several forms of hormonal therapy:
- Orchiectomy is surgery to remove the testicles. Because the testicles make most of the body's testosterone, after orchiectomy testosterone levels drop. Now the prostate not only lacks the testosterone stimulus to produce DHT, but also it does not have enough testosterone to transform into DHT.
- Antiandrogens are medications such as flutamide, bicalutamide, nilutamide, and cyproterone acetate which directly block the actions of testosterone and DHT within prostate cancer cells.
- Medications which block the production of adrenal androgens such as DHEA include ketoconazole and aminoglutethimide. Because the adrenal glands only make about 5% of the body's androgens, these medications are generally used only in combination with other methods that can block the 95% of androgens made by the testicles. These combined methods are called total androgen blockade (TAB). TAB can also be achieved using antiandrogens.
- GnRH action can be interrupted in one of two ways. GnRH antagonists suppress the production of LH directly, while GnRH agonists suppress LH through the process of downregulation after an initial stimulation effect. Abarelix is an example of a GnRH antagonist, while the GnRH agonists include leuprolide, goserelin, triptorelin, and buserelin. Initially, GnRH agonists increase the production of LH. However, because the constant supply of the medication does not match the body's natural production rhythm, production of both LH and GnRH decreases after a few weeks.[13]
- A very recent Trial I study (N=21) found that Abiraterone Acetate caused dramatic reduction in PSA levels and Tumor sizes in aggressive end-stage prostate cancer for 70% of patients. This is prostate cancer that resists all other treatments (e.g., castration, other hormones, etc.). Officially the impacts on life-span are not yet known because subjects have not been taking the drug very long. Larger Trial III Clinical Studies are in the works. If successful an approved treatment is hoped for around 2011.[14][15]
The most successful hormonal treatments are orchiectomy and GnRH agonists. Despite their higher cost, GnRH agonists are often chosen over orchiectomy for cosmetic and emotional reasons. Eventually, total androgen blockade may prove to be better than orchiectomy or GnRH agonists used alone.
Each treatment has disadvantages which limit its use in certain circumstances. Although orchiectomy is a low-risk surgery, the psychological impact of removing the testicles can be significant. The loss of testosterone also causes hot flashes, weight gain, loss of libido, enlargement of the breasts (gynecomastia), impotence and osteoporosis. GnRH agonists eventually cause the same side effects as orchiectomy but may cause worse symptoms at the beginning of treatment. When GnRH agonists are first used, testosterone surges can lead to increased bone pain from metastatic cancer, so antiandrogens or abarelix are often added to blunt these side effects. Estrogens are not commonly used because they increase the risk for cardiovascular disease and blood clots. The antiandrogens do not generally cause impotence and usually cause less loss of bone and muscle mass. Ketoconazole can cause liver damage with prolonged use, and aminoglutethimide can cause skin rashes.
References
- ↑ Ornish, D (2005). "Intensive lifestyle changes may affect the progression of prostate cancer". J Urol. 174 (3): 1065–70. PMID 16094059. Unknown parameter
|coauthors=ignored (help) - ↑ Frattaroli, J (2008). "Clinical events in Prostate CAncer Lifestyle Trial: Results from two years of follow-up". Urology. epub ahead of print. PMID 18602144. Unknown parameter
|coauthors=ignored (help); Unknown parameter|month=ignored (help) - ↑ Pantuck, AJ (2006). "Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer". Clin Cancer Res. 12 (13): 4018–26. PMID 16818701. Unknown parameter
|coauthors=ignored (help) - ↑ Kumar, NB (2004). "The specific role of isoflavones in reducing prostate cancer risk". Prostate. 59 (2): 141–7. PMID 15042614. Unknown parameter
|coauthors=ignored (help) - ↑ Yarnell, E (1999). "A naturopathic approach to prostate cancer. Part 2: Guidelines for treatment and prevention". Altern Complemen Ther. 5 (6): 360–8.
- ↑ Nag, S (1999). "American Brachytherapy Society Recommendations for Transperineal Permanent Brachytherapy of Prostate Cancer". Int. J. Rad. Onc. Biol. Phys. 44 (4): 789–799. ?. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) Review. - ↑ Perez, CA (1993). "Localized carcinoma of the prostate (stages T1B, T1C, T2, and T3). Review of management with external beam radiation therapy". Cancer. 72 (11): 3156–73. doi:10.1002/1097-0142(19931201)72:11<3156::AID-CNCR2820721106>3.0.CO;2-G. PMID 7694785. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) Review. - ↑ D'Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW (2004). "6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial". JAMA. 292 (7): 821–7. doi:10.1001/jama.292.7.821. PMID 15315996.
- ↑ American Cancer Society: Radiation Treatment
- ↑ Lawton, CA (1991). "Long-term treatment sequelae following external beam irradiation for adenocarcinoma of the prostate: analysis of RTOG studies 7506 and 7706". Int J Radiat Oncol Biol Phys. 21 (4): 935–9. PMID 1917622. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) - ↑ Brenner, DJ (2000). "Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery". Cancer. 88 (2): 398–406. doi:10.1002/(SICI)1097-0142(20000115)88:2<398::AID-CNCR22>3.0.CO;2-V. PMID 10640974. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) - ↑ Robson, M (1996). "How is androgen-dependent metastatic prostate cancer best treated?". Hematol Oncol Clin North Am. 10 (3): 727–47. doi:10.1016/S0889-8588(05)70364-6. PMID 8773508. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) Review. - ↑ Loblaw, DA (2004). "American Society of Clinical Oncology recommendations for the initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer". J Clin Oncol. 22 (14): 2927–41. doi:10.1200/JCO.2004.04.579. PMID 15184404. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) Erratum in: J Clin Oncol. 2004 November 1;22(21):4435. - ↑ de Bono, Johann (2004). "Phase I Clinical Trial of a Selective Inhibitor of CYP17, Abiraterone Acetate, Confirms That Castration-Resistant Prostate Cancer Commonly Remains Hormone Driven". J Clin Oncol: online. doi:10.1200/JCO.2007.15.9749. PMID 15184404. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help) Erratum in: J Clin Oncol. Early Release, published ahead of print July 21, 2008 - ↑ Richard Warry (July 22, 2008). "Drug for deadly prostate cancer". BBC. Retrieved 2008-07-23.