Artemether and lumefantrin: Difference between revisions
No edit summary |
No edit summary |
||
| Line 86: | Line 86: | ||
*Hypersensitivity reactions including urticaria and angioedema. Serious skin reactions (bullous eruption) have been rarely reported. | *Hypersensitivity reactions including urticaria and angioedema. Serious skin reactions (bullous eruption) have been rarely reported. | ||
|drugInteractions=====Rifampin==== | |drugInteractions=====Rifampin==== | ||
Oral administration of [[rifampin]], a strong [[CYP3A4]] inducer, with Coartem Tablets resulted in significant decreases in exposure to artemether, dihydroartemisinin (DHA, metabolite of artemether) and [[lumefantrine]] by 89%, 85% and 68%, respectively, when compared to exposure values after Coartem Tablets alone. Concomitant use of strong inducers of [[CYP3A4]] such as [[rifampin]], [[carbamazepine]], [[phenytoin]] and [[St. John's wort]] is contraindicated with Coartem Tablets. | *Oral administration of [[rifampin]], a strong [[CYP3A4]] inducer, with Coartem Tablets resulted in significant decreases in exposure to artemether, dihydroartemisinin (DHA, metabolite of artemether) and [[lumefantrine]] by 89%, 85% and 68%, respectively, when compared to exposure values after Coartem Tablets alone. Concomitant use of strong inducers of [[CYP3A4]] such as [[rifampin]], [[carbamazepine]], [[phenytoin]] and [[St. John's wort]] is contraindicated with Coartem Tablets. | ||
====Ketoconazole==== | ====Ketoconazole==== | ||
Concurrent oral administration of [[ketoconazole]], a potent [[CYP3A4]] inhibitor, with a single dose of Coartem Tablets resulted in a moderate increase in exposure to artemether, DHA, and lumefantrine in a study of 15 healthy subjects. No dose adjustment of Coartem Tablets is necessary when administered with [[ketoconazole]] or other potent [[CYP3A4]] inhibitors. However, due to the potential for increased concentrations of lumefantrine which could lead to [[QT prolongation]], Coartem Tablets should be used cautiously with drugs that inhibit [[CYP3A4]]. | *Concurrent oral administration of [[ketoconazole]], a potent [[CYP3A4]] inhibitor, with a single dose of Coartem Tablets resulted in a moderate increase in exposure to artemether, DHA, and lumefantrine in a study of 15 healthy subjects. No dose adjustment of Coartem Tablets is necessary when administered with [[ketoconazole]] or other potent [[CYP3A4]] inhibitors. However, due to the potential for increased concentrations of lumefantrine which could lead to [[QT prolongation]], Coartem Tablets should be used cautiously with drugs that inhibit [[CYP3A4]]. | ||
====Antiretroviral Drugs==== | ====Antiretroviral Drugs==== | ||
Both artemether and [[lumefantrine]] are metabolized by [[CYP3A4]]. [[Antiretroviral drugs]], such as [[protease inhibitors]] and [[non-nucleoside reverse transcriptase inhibitors]], are known to have variable patterns of inhibition, induction or competition for [[CYP3A4]]. Therefore, the effects of antiretroviral drugs on the exposure to artemether, DHA, and [[lumefantrine]] are also variable [see Clinical Pharmacology (12.3)]. Coartem Tablets should be used cautiously in patients on antiretroviral drugs because decreased artemether, DHA, and/or [[lumefantrine]] concentrations may result in a decrease of antimalarial efficacy of Coartem Tablets, and increased lumefantrine concentrations may cause [[QT prolongation]]. | *Both artemether and [[lumefantrine]] are metabolized by [[CYP3A4]]. [[Antiretroviral drugs]], such as [[protease inhibitors]] and [[non-nucleoside reverse transcriptase inhibitors]], are known to have variable patterns of inhibition, induction or competition for [[CYP3A4]]. Therefore, the effects of antiretroviral drugs on the exposure to artemether, DHA, and [[lumefantrine]] are also variable [see Clinical Pharmacology (12.3)]. Coartem Tablets should be used cautiously in patients on antiretroviral drugs because decreased artemether, DHA, and/or [[lumefantrine]] concentrations may result in a decrease of antimalarial efficacy of Coartem Tablets, and increased lumefantrine concentrations may cause [[QT prolongation]]. | ||
====Prior Use of Mefloquine==== | ====Prior Use of Mefloquine==== | ||
Administration of three doses of [[mefloquine]] followed 12 hours later by a 6-dose regimen of Coartem Tablets in 14 healthy volunteers demonstrated no effect of [[mefloquine]] on plasma concentrations of artemether or the artemether/DHA ratio. However, exposure to [[lumefantrine]] was reduced, possibly due to lower absorption secondary to a [[mefloquine]]-induced decrease in bile production. Patients should be monitored for decreased efficacy and food consumption should be encouraged with administration of Coartem Tablets. | *Administration of three doses of [[mefloquine]] followed 12 hours later by a 6-dose regimen of Coartem Tablets in 14 healthy volunteers demonstrated no effect of [[mefloquine]] on plasma concentrations of artemether or the artemether/DHA ratio. However, exposure to [[lumefantrine]] was reduced, possibly due to lower absorption secondary to a [[mefloquine]]-induced decrease in bile production. Patients should be monitored for decreased efficacy and food consumption should be encouraged with administration of Coartem Tablets. | ||
====Hormonal Contraceptives==== | ====Hormonal Contraceptives==== | ||
In vitro, the metabolism of [[ethinyl estradiol]] and [[levonorgestrel]] was not induced by artemether, DHA, or [[lumefantrine]]. However, artemether has been reported to weakly induce, in humans, the activity of [[CYP2C19]], [[CYP2B6]], and [[CYP3A]]. Therefore, Coartem Tablets may potentially reduce the effectiveness of [[hormonal contraceptives]]. Patients using oral, transdermal patch, or other systemic hormonal contraceptives should be advised to use an additional non-hormonal method of birth control. | *In vitro, the metabolism of [[ethinyl estradiol]] and [[levonorgestrel]] was not induced by artemether, DHA, or [[lumefantrine]]. However, artemether has been reported to weakly induce, in humans, the activity of [[CYP2C19]], [[CYP2B6]], and [[CYP3A]]. Therefore, Coartem Tablets may potentially reduce the effectiveness of [[hormonal contraceptives]]. Patients using oral, transdermal patch, or other systemic hormonal contraceptives should be advised to use an additional non-hormonal method of birth control. | ||
====CYP2D6 Substrates==== | ====CYP2D6 Substrates==== | ||
Lumefantrine inhibits [[CYP2D6]] in vitro. Administration of Coartem Tablets with drugs that are metabolized by [[CYP2D6]] may significantly increase plasma concentrations of the co-administered drug and increase the risk of adverse effects. Many of the drugs metabolized by [[CYP2D6]] can prolong the [[QT interval]] and should not be administered with Coartem Tablets due to the potential additive effect on the [[QT interval]] (e.g., [[flecainide]], [[imipramine]], [[amitriptyline]], [[clomipramine]]) | *Lumefantrine inhibits [[CYP2D6]] in vitro. Administration of Coartem Tablets with drugs that are metabolized by [[CYP2D6]] may significantly increase plasma concentrations of the co-administered drug and increase the risk of adverse effects. Many of the drugs metabolized by [[CYP2D6]] can prolong the [[QT interval]] and should not be administered with Coartem Tablets due to the potential additive effect on the [[QT interval]] (e.g., [[flecainide]], [[imipramine]], [[amitriptyline]], [[clomipramine]]) | ||
====Sequential Use of Quinine==== | ====Sequential Use of Quinine==== | ||
A single dose of intravenous [[quinine]] (10 mg/kg bodyweight) concurrent with the final dose of a 6-dose regimen of Coartem Tablets demonstrated no effect of intravenous [[quinine]] on the systemic exposure of DHA or [[lumefantrine]]. [[Quinine]] exposure was also not altered. Exposure to artemether was decreased. This decrease in artemether exposure is not thought to be clinically significant. However, [[quinine]] and other drugs that prolong the [[QT interval]] should be used cautiously following treatment with Coartem Tablets due to the long elimination half-life of [[lumefantrine]] and the potential for additive [[QT effects]]; ECG monitoring is advised if use of drugs that prolong the [[QT interval]] is medically required. | *A single dose of intravenous [[quinine]] (10 mg/kg bodyweight) concurrent with the final dose of a 6-dose regimen of Coartem Tablets demonstrated no effect of intravenous [[quinine]] on the systemic exposure of DHA or [[lumefantrine]]. [[Quinine]] exposure was also not altered. Exposure to artemether was decreased. This decrease in artemether exposure is not thought to be clinically significant. However, [[quinine]] and other drugs that prolong the [[QT interval]] should be used cautiously following treatment with Coartem Tablets due to the long elimination half-life of [[lumefantrine]] and the potential for additive [[QT effects]]; ECG monitoring is advised if use of drugs that prolong the [[QT interval]] is medically required. | ||
====Interaction with Drugs that are Known to Prolong the QT Interval==== | ====Interaction with Drugs that are Known to Prolong the QT Interval==== | ||
Coartem is to be used with caution when co-administered with drugs that may cause prolonged [[QT interval]] such as [[antiarrhythmics]] of classes IA and III, [[neuroleptics]] and [[antidepressant agents]], certain [[antibiotics]] including some agents of the following classes: [[macrolides]], [[fluoroquinolones]], [[imidazole]], and [[triazole]] [[antifungal agents]]. | *Coartem is to be used with caution when co-administered with drugs that may cause prolonged [[QT interval]] such as [[antiarrhythmics]] of classes IA and III, [[neuroleptics]] and [[antidepressant agents]], certain [[antibiotics]] including some agents of the following classes: [[macrolides]], [[fluoroquinolones]], [[imidazole]], and [[triazole]] [[antifungal agents]]. | ||
|FDAPregCat=C | |||
|useInPregnancyFDA=Safety data from an observational pregnancy study of approximately 500 pregnant women who were exposed to Coartem Tablets (including a third of patients who were exposed in the first trimester), and published data of over 1,000 pregnant patients who were exposed to artemisinin derivatives, did not show an increase in adverse pregnancy outcomes or teratogenic effects over background rate. | |||
The efficacy of Coartem Tablets in the treatment of acute, uncomplicated malaria in pregnant women has not been established. | |||
Coartem Tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | |||
Pregnant rats dosed during the period of organogenesis at or higher than a dose of about half the highest clinical dose of 1120 mg artemether-lumefantrine per day (based on body surface area comparisons), showed increases in fetal loss, early resorptions and post implantation loss. No adverse effects were observed in animals dosed at about one-third the highest clinical dose. Similarly, dosing in pregnant rabbits at about three times the clinical dose (based on body surface area comparisons) resulted in abortions, preimplantation loss, post implantation loss and decreases in the number of live fetuses. No adverse reproductive effects were detected in rabbits at two times the clinical dose. Embryo-fetal loss is a significant reproductive toxicity. Other artemisinins are known to be embryotoxic in animals. However, because metabolic profiles in animals and humans are dissimilar, artemether exposures in animals may not be predictive of human exposures [see Nonclinical Toxicology (13.2)]. These data cannot rule out an increased risk for early pregnancy loss or fetal defects in humans. | |||
|useInNursing=It is not known whether artemether or lumefantrine is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Coartem Tablets are administered to a nursing woman. Animal data suggest both artemether and lumefantrine are excreted into breast milk. The benefits of breastfeeding to mother and infant should be weighed against potential risk from infant exposure to artemether and lumefantrine through breast milk. | |||
|useInPed=The safety and effectiveness of Coartem Tablets have been established for the treatment of acute, uncomplicated malaria in studies involving pediatric patients weighing 5 kg or more [see Clinical Studies (14.1)]. The safety and efficacy have not been established in pediatric patients who weigh less than 5 kg. Children from non-endemic countries were not included in clinical trials. | |||
|useInGeri=Clinical studies of Coartem Tablets did not include sufficient numbers of subjects aged 65 years and over to determine they respond differently from younger subjects. In general, the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy in elderly patients should be considered when prescribing Coartem Tablets. | |||
|useInRenalImpair=No specific pharmacokinetic studies have been performed in patients with either hepatic or renal impairment. Coartem Tablets have not been studied for efficacy and safety in patients with severe hepatic and/or renal impairment. Based on the pharmacokinetic data in 16 healthy subjects showing no or insignificant renal excretion of lumefantrine, artemether and DHA, no dose adjustment for the use of Coartem in patients with renal impairment is advised. No dosage adjustment is necessary in patients with mild to moderate hepatic impairment [see Dosage and Administration (2.4) and Warnings and Precautions (5.6)]. | |||
|useInHepaticImpair=No specific pharmacokinetic studies have been performed in patients with either hepatic or renal impairment. Coartem Tablets have not been studied for efficacy and safety in patients with severe hepatic and/or renal impairment. Based on the pharmacokinetic data in 16 healthy subjects showing no or insignificant renal excretion of lumefantrine, artemether and DHA, no dose adjustment for the use of Coartem in patients with renal impairment is advised. No dosage adjustment is necessary in patients with mild to moderate hepatic impairment [see Dosage and Administration (2.4) and Warnings and Precautions (5.6)]. | |||
|overdose=*There is no information on overdoses of Coartem Tablets higher than the doses recommended for treatment. | |||
*In cases of suspected overdosage, symptomatic and supportive therapy, which would include ECG and blood electrolyte monitoring, should be given as appropriate. | |||
|drugBox={{Drugbox2 | |||
| Verifiedfields = changed | |||
| Watchedfields = changed | |||
| verifiedrevid = 457818137 | |||
| IUPAC_name = (3''R'',5a''S'',6''R'',8a''S'',9''R'',10''S'',12''R'',12a''R'')-10-methoxy-3,6,9-trimethyldecahydro-12''H''-3,12-epoxy[1,2]dioxepino[4,3-''i'']-2-benzopyran | |||
| image = Artemether Structure.png | |||
<!--Clinical data--> | |||
| tradename = | |||
| Drugs.com = {{drugs.com|international|artemether}} | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = C | |||
| pregnancy_category = | |||
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled--> | |||
| legal_CA = <!-- Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| legal_UK = POM | |||
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> | |||
| legal_status = | |||
| routes_of_administration = Oral | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = | |||
| elimination_half-life = | |||
| excretion = | |||
<!--Identifiers--> | |||
| CAS_number_Ref = {{cascite|changed|??}} | |||
| CAS_number = 71963-77-4 | |||
| ATC_prefix = P01 | |||
| ATC_suffix = BE02 | |||
| ATC_supplemental = | |||
| PubChem = 68911 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB06697 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 62138 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = C7D6T3H22J | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D02483 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 195280 | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 1237051 | |||
<!--Chemical data--> | |||
| chemical_formula = | |||
| C=16 | H=26 | O=5 | |||
| molecular_weight = 298.374 g/mol | |||
| smiles = C[C@@H]1CC[C@@H]3C42OO[C@](C)(CC[C@@H]12)O[C@H]4O[C@H](OC)[C@@H]3C | |||
| InChI = 1/C16H26O5/c1-9-5-6-12-10(2)13(17-4)18-14-16(12)11(9)7-8-15(3,19-14)20-21-16/h9-14H,5-8H2,1-4H3/t9-,10-,11+,12+,13+,14-,15-,16?/m1/s1 | |||
| InChIKey = SXYIRMFQILZOAM-LJXHFVHTBB | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C16H26O5/c1-9-5-6-12-10(2)13(17-4)18-14-16(12)11(9)7-8-15(3,19-14)20-21-16/h9-14H,5-8H2,1-4H3/t9-,10-,11+,12+,13+,14-,15-,16-/m1/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = SXYIRMFQILZOAM-HVNFFKDJSA-N | |||
}} | |||
|mechAction=Coartem Tablets, a fixed dose combination of artemether and lumefantrine in the ratio of 1:6, is an antimalarial agent. | |||
|structure=Artemether has the empirical formula C16H26O5 with a molecular weight of 298.4, and the following structural formula: | |||
[[file:Artemether Structure.png|none|300px]] | |||
[[Lumefantrin]] has the empirical formula C30H32Cl3NO with a molecular weight of 528.9, and the following structural formula: | |||
[[file:Lumefantrine Structure.png|none|300px]] | |||
|alcohol=Alcohol-Artemether and lumefantrin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Artemether and lumefantrin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 21:06, 4 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Artemether and lumefantrin is an antimanlaric that is FDA approved for the treatment of acute, uncomplicated malaria infections due to Plasmodium falciparum in patients of 5 kg bodyweight and above. Common adverse reactions include headache, anorexia, dizziness, asthenia, arthralgia and myalgia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Artemether and lumefantrin FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Artemether and lumefantrin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Artemether and lumefantrin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Artemether and lumefantrin FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Artemether and lumefantrin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Artemether and lumefantrin in pediatric patients.
Contraindications
Hypersensitivity
- Known hypersensitivity to artemether, lumefantrine, or to any of the excipients of Coartem Tablets.
Strong CYP3A4 Inducers
- Co-administration of strong inducers of CYP3A4 such as rifampin, carbamazepine, phenytoin and St. John's wort with Coartem Tablets can result in decreased concentrations of artemether and/or lumefantrine and loss of antimalarial efficacy.
Warnings
Prolongation of the QT Interval
Some antimalarials (e.g., halofantrine, quinine, quinidine) including Coartem Tablets have been associated with prolongation of the QT interval on the electrocardiogram. Coartem Tablets should be avoided in patients:
- With congenital prolongation of the QT interval (e.g., long QT syndrome) or any other clinical condition known to prolong the QTc interval such as patients with a history of symptomatic cardiac arrhythmias, with clinically relevant bradycardia or with severe cardiac disease.
- With a family history of congenital prolongation of the QT interval or sudden death.
- With known disturbances of electrolyte balance, e.g., hypokalemia or hypomagnesemia.
- Receiving other medications that prolong the QT interval, such as class IA (quinidine, procainamide, disopyramide), or class III (amiodarone, sotalol) antiarrhythmic agents; antipsychotics (pimozide, ziprasidone); antidepressants; certain antibiotics (macrolide antibiotics, fluoroquinolone antibiotics, imidazole, and triazole antifungal agents).
- Receiving medications that are metabolized by the cytochrome enzyme CYP2D6 which also have cardiac effects (e.g., flecainide, imipramine, amitriptyline, clomipramine).
Use of QT Prolonging Drugs and Other Antimalarials
- Halofantrine and Coartem Tablets should not be administered within one month of each other due to the long elimination half-life of lumefantrine (3-6 days) and potential additive effects on the QT interval.
- Antimalarials should not be given concomitantly with Coartem Tablets, unless there is no other treatment option, due to limited safety data.
- Drugs that prolong the QT interval, including antimalarials such as quinine and quinidine, should be used cautiously following Coartem Tablets, due to the long elimination half-life of lumefantrine (3-6 days) and the potential for additive effects on the QT interval; ECG monitoring is advised if use of drugs that prolong the QT interval is medically required.
- If mefloquine is administered immediately prior to Coartem Tablets there may be a decreased exposure to lumefantrine, possibly due to a mefloquine-induced decrease in bile production. Therefore, patients should be monitored for decreased efficacy and food consumption should be encouraged while taking Coartem Tablets.
Drug Interactions with CYP3A4
- When Coartem Tablets are co-administered with substrates of CYP3A4 it may result in decreased concentrations of the substrate and potential loss of substrate efficacy. When Coartem Tablets are co-administered with an inhibitor of CYP3A4, including grapefruit juice it may result in increased concentrations of artemether and/or lumefantrine and potentiate QT prolongation. When Coartem Tablets are co-administered with inducers of CYP3A4 it may result in decreased concentrations of artemether and/or lumefantrine and loss of antimalarial efficacy.
- Drugs that have a mixed effect on CYP3A4, especially antiretroviral drugs such as HIV protease inhibitors and non-nucleoside reverse transcriptase inhibitors, and those that have an effect on the QT interval should be used with caution in patients taking Coartem Tablets.
- Coartem Tablets may reduce the effectiveness of hormonal contraceptives. Therefore, patients using oral, transdermal patch, or other systemic hormonal contraceptives should be advised to use an additional non-hormonal method of birth control.
Drug Interactions with CYP2D6
- Administration of Coartem Tablets with drugs that are metabolized by CYP2D6 may significantly increase plasma concentrations of the co-administered drug and increase the risk of adverse effects. Many of the drugs metabolized by CYP2D6 can prolong the QT interval and should not be administered with Coartem Tablets due to the potential additive effect on the QT interval (e.g., flecainide, imipramine, amitriptyline, clomipramine).
Recrudescence
- Food enhances absorption of artemether and lumefantrine following administration of Coartem Tablets.
- Patients who remain averse to food during treatment should be closely monitored as the risk of recrudescence may be greater. In the event of recrudescent P. falciparum infection after treatment with Coartem Tablets, patients should be treated with a different antimalarial drug.
Hepatic and Renal Impairment
- Coartem Tablets have not been studied for efficacy and safety in patients with severe hepatic and/or renal impairment.
Plasmodium vivax Infection
- Coartem Tablets have been shown in limited data (43 patients) to be effective in treating the erythrocytic stage of P. vivax infection. However, relapsing malaria caused by P. vivax requires additional treatment with other antimalarial agents to achieve radical cure i.e., eradicate any hypnozoites forms that may remain dormant in the liver.
Adverse Reactions
Clinical Trials Experience
Serious Adverse Reactions
The following serious and otherwise important adverse reactions are discussed in greater detail in other sections of labeling:
Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rate observed in practice.
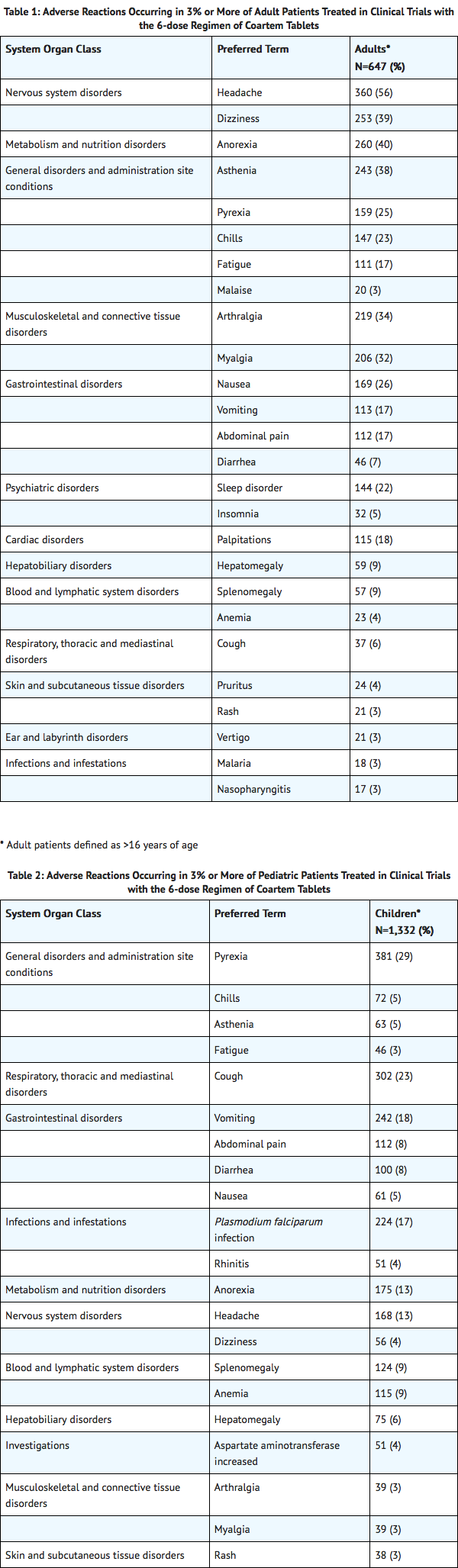
The data described below reflect exposure to a 6-dose regimen of Coartem Tablets in 1,979 patients including 647 adults (older than 16 years) and 1,332 children (16 years and younger). For the 6-dose regimen, Coartem Tablets was studied in active-controlled (366 patients) and non-controlled, open-label trials (1,613 patients). The 6-dose Coartem Tablets population was patients with malaria between ages 2 months and 71 years: 67% (1,332) were 16 years and younger and 33% (647) were older than 16 years. Males represented 73% and 53% of the adult and pediatric populations, respectively. The majority of adult patients were enrolled in studies in Thailand, while the majority of pediatric patients were enrolled in Africa.
Tables 1 and 2 show the most frequently reported adverse reactions (вЙ•3%) in adults and children respectively who received the 6-dose regimen of Coartem Tablets. Adverse reactions collected in clinical trials included signs and symptoms at baseline but only treatment emergent adverse events, defined as events that appeared or worsened after the start of treatment, are presented below. In adults, the most frequently reported adverse reactions were headache, anorexia, dizziness, and asthenia. In children, the adverse reactions were pyrexia, cough, vomiting, anorexia, and headache. Most adverse reactions were mild, did not lead to discontinuation of study medication, and resolved.
In limited comparative studies, the adverse reaction profile of Coartem Tablets appeared similar to that of another antimalarial regimen.
Discontinuation of Coartem Tablets due to adverse drug reactions occurred in 1.1% of patients treated with the 6-dose regimen overall: 0.2% (1/647) in adults and 1.6% (21/1,332) in children.
Clinically significant adverse reactions reported in adults and/or children treated with the 6-dose regimen of Coartem Tablets which occurred in clinical studies at <3% regardless of causality are listed below:
- Blood and lymphatic system disorders: eosinophilia
- Ear and labyrinth disorders: tinnitus
- Eye disorders: conjunctivitis
- Gastrointestinal disorders: constipation, dyspepsia, dysphagia, peptic ulcer
- General disorders: gait disturbance
- Infections and infestations: abscess, acrodermatitis, bronchitis, ear infection, gastroenteritis, helminthic infection, hookworm infection, impetigo, influenza, lower respiratory tract infection, malaria, nasopharyngitis, oral herpes, pneumonia, respiratory tract infection, subcutaneous abscess, upper respiratory tract infection, urinary tract infection
- Investigations: alanine aminotransferase increased, aspartate aminotransferase increased, hematocrit decreased, lymphocyte morphology abnormal, platelet count decreased, platelet count increased, white blood cell count decreased, white blood cell count increased
- Metabolism and nutrition disorders: hypokalemia
- Musculoskeletal and connective tissue disorders: back pain
- Nervous system disorders: ataxia, clonus, fine motor delay, hyperreflexia, hypoaesthesia, nystagmus, tremor.
- Psychiatric disorders: agitation, mood swings
- Renal and urinary disorders: hematuria, proteinuria
- Respiratory, thoracic and mediastinal disorders: asthma, pharyngo-laryngeal pain
- Skin and subcutaneous tissue disorders: urticaria
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Coartem Tablets. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Hypersensitivity reactions including urticaria and angioedema. Serious skin reactions (bullous eruption) have been rarely reported.
Drug Interactions
Rifampin
- Oral administration of rifampin, a strong CYP3A4 inducer, with Coartem Tablets resulted in significant decreases in exposure to artemether, dihydroartemisinin (DHA, metabolite of artemether) and lumefantrine by 89%, 85% and 68%, respectively, when compared to exposure values after Coartem Tablets alone. Concomitant use of strong inducers of CYP3A4 such as rifampin, carbamazepine, phenytoin and St. John's wort is contraindicated with Coartem Tablets.
Ketoconazole
- Concurrent oral administration of ketoconazole, a potent CYP3A4 inhibitor, with a single dose of Coartem Tablets resulted in a moderate increase in exposure to artemether, DHA, and lumefantrine in a study of 15 healthy subjects. No dose adjustment of Coartem Tablets is necessary when administered with ketoconazole or other potent CYP3A4 inhibitors. However, due to the potential for increased concentrations of lumefantrine which could lead to QT prolongation, Coartem Tablets should be used cautiously with drugs that inhibit CYP3A4.
Antiretroviral Drugs
- Both artemether and lumefantrine are metabolized by CYP3A4. Antiretroviral drugs, such as protease inhibitors and non-nucleoside reverse transcriptase inhibitors, are known to have variable patterns of inhibition, induction or competition for CYP3A4. Therefore, the effects of antiretroviral drugs on the exposure to artemether, DHA, and lumefantrine are also variable [see Clinical Pharmacology (12.3)]. Coartem Tablets should be used cautiously in patients on antiretroviral drugs because decreased artemether, DHA, and/or lumefantrine concentrations may result in a decrease of antimalarial efficacy of Coartem Tablets, and increased lumefantrine concentrations may cause QT prolongation.
Prior Use of Mefloquine
- Administration of three doses of mefloquine followed 12 hours later by a 6-dose regimen of Coartem Tablets in 14 healthy volunteers demonstrated no effect of mefloquine on plasma concentrations of artemether or the artemether/DHA ratio. However, exposure to lumefantrine was reduced, possibly due to lower absorption secondary to a mefloquine-induced decrease in bile production. Patients should be monitored for decreased efficacy and food consumption should be encouraged with administration of Coartem Tablets.
Hormonal Contraceptives
- In vitro, the metabolism of ethinyl estradiol and levonorgestrel was not induced by artemether, DHA, or lumefantrine. However, artemether has been reported to weakly induce, in humans, the activity of CYP2C19, CYP2B6, and CYP3A. Therefore, Coartem Tablets may potentially reduce the effectiveness of hormonal contraceptives. Patients using oral, transdermal patch, or other systemic hormonal contraceptives should be advised to use an additional non-hormonal method of birth control.
CYP2D6 Substrates
- Lumefantrine inhibits CYP2D6 in vitro. Administration of Coartem Tablets with drugs that are metabolized by CYP2D6 may significantly increase plasma concentrations of the co-administered drug and increase the risk of adverse effects. Many of the drugs metabolized by CYP2D6 can prolong the QT interval and should not be administered with Coartem Tablets due to the potential additive effect on the QT interval (e.g., flecainide, imipramine, amitriptyline, clomipramine)
Sequential Use of Quinine
- A single dose of intravenous quinine (10 mg/kg bodyweight) concurrent with the final dose of a 6-dose regimen of Coartem Tablets demonstrated no effect of intravenous quinine on the systemic exposure of DHA or lumefantrine. Quinine exposure was also not altered. Exposure to artemether was decreased. This decrease in artemether exposure is not thought to be clinically significant. However, quinine and other drugs that prolong the QT interval should be used cautiously following treatment with Coartem Tablets due to the long elimination half-life of lumefantrine and the potential for additive QT effects; ECG monitoring is advised if use of drugs that prolong the QT interval is medically required.
Interaction with Drugs that are Known to Prolong the QT Interval
- Coartem is to be used with caution when co-administered with drugs that may cause prolonged QT interval such as antiarrhythmics of classes IA and III, neuroleptics and antidepressant agents, certain antibiotics including some agents of the following classes: macrolides, fluoroquinolones, imidazole, and triazole antifungal agents.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C Safety data from an observational pregnancy study of approximately 500 pregnant women who were exposed to Coartem Tablets (including a third of patients who were exposed in the first trimester), and published data of over 1,000 pregnant patients who were exposed to artemisinin derivatives, did not show an increase in adverse pregnancy outcomes or teratogenic effects over background rate.
The efficacy of Coartem Tablets in the treatment of acute, uncomplicated malaria in pregnant women has not been established.
Coartem Tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnant rats dosed during the period of organogenesis at or higher than a dose of about half the highest clinical dose of 1120 mg artemether-lumefantrine per day (based on body surface area comparisons), showed increases in fetal loss, early resorptions and post implantation loss. No adverse effects were observed in animals dosed at about one-third the highest clinical dose. Similarly, dosing in pregnant rabbits at about three times the clinical dose (based on body surface area comparisons) resulted in abortions, preimplantation loss, post implantation loss and decreases in the number of live fetuses. No adverse reproductive effects were detected in rabbits at two times the clinical dose. Embryo-fetal loss is a significant reproductive toxicity. Other artemisinins are known to be embryotoxic in animals. However, because metabolic profiles in animals and humans are dissimilar, artemether exposures in animals may not be predictive of human exposures [see Nonclinical Toxicology (13.2)]. These data cannot rule out an increased risk for early pregnancy loss or fetal defects in humans.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Artemether and lumefantrin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Artemether and lumefantrin during labor and delivery.
Nursing Mothers
It is not known whether artemether or lumefantrine is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Coartem Tablets are administered to a nursing woman. Animal data suggest both artemether and lumefantrine are excreted into breast milk. The benefits of breastfeeding to mother and infant should be weighed against potential risk from infant exposure to artemether and lumefantrine through breast milk.
Pediatric Use
The safety and effectiveness of Coartem Tablets have been established for the treatment of acute, uncomplicated malaria in studies involving pediatric patients weighing 5 kg or more [see Clinical Studies (14.1)]. The safety and efficacy have not been established in pediatric patients who weigh less than 5 kg. Children from non-endemic countries were not included in clinical trials.
Geriatic Use
Clinical studies of Coartem Tablets did not include sufficient numbers of subjects aged 65 years and over to determine they respond differently from younger subjects. In general, the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy in elderly patients should be considered when prescribing Coartem Tablets.
Gender
There is no FDA guidance on the use of Artemether and lumefantrin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Artemether and lumefantrin with respect to specific racial populations.
Renal Impairment
No specific pharmacokinetic studies have been performed in patients with either hepatic or renal impairment. Coartem Tablets have not been studied for efficacy and safety in patients with severe hepatic and/or renal impairment. Based on the pharmacokinetic data in 16 healthy subjects showing no or insignificant renal excretion of lumefantrine, artemether and DHA, no dose adjustment for the use of Coartem in patients with renal impairment is advised. No dosage adjustment is necessary in patients with mild to moderate hepatic impairment [see Dosage and Administration (2.4) and Warnings and Precautions (5.6)].
Hepatic Impairment
No specific pharmacokinetic studies have been performed in patients with either hepatic or renal impairment. Coartem Tablets have not been studied for efficacy and safety in patients with severe hepatic and/or renal impairment. Based on the pharmacokinetic data in 16 healthy subjects showing no or insignificant renal excretion of lumefantrine, artemether and DHA, no dose adjustment for the use of Coartem in patients with renal impairment is advised. No dosage adjustment is necessary in patients with mild to moderate hepatic impairment [see Dosage and Administration (2.4) and Warnings and Precautions (5.6)].
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Artemether and lumefantrin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Artemether and lumefantrin in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Artemether and lumefantrin Administration in the drug label.
Monitoring
There is limited information regarding Artemether and lumefantrin Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Artemether and lumefantrin and IV administrations.
Overdosage
- There is no information on overdoses of Coartem Tablets higher than the doses recommended for treatment.
- In cases of suspected overdosage, symptomatic and supportive therapy, which would include ECG and blood electrolyte monitoring, should be given as appropriate.
Pharmacology
| |
Artemether and lumefantrin
| |
| Systematic (IUPAC) name | |
| (3R,5aS,6R,8aS,9R,10S,12R,12aR)-10-methoxy-3,6,9-trimethyldecahydro-12H-3,12-epoxy[1,2]dioxepino[4,3-i]-2-benzopyran | |
| Identifiers | |
| CAS number | |
| ATC code | P01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 298.374 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status | |
| Routes | Oral |
Mechanism of Action
Coartem Tablets, a fixed dose combination of artemether and lumefantrine in the ratio of 1:6, is an antimalarial agent.
Structure
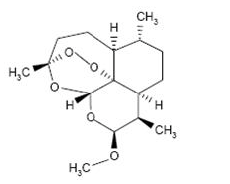
Artemether has the empirical formula C16H26O5 with a molecular weight of 298.4, and the following structural formula:

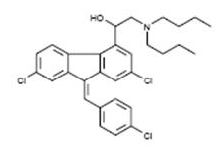
Lumefantrin has the empirical formula C30H32Cl3NO with a molecular weight of 528.9, and the following structural formula:
Pharmacodynamics
There is limited information regarding Artemether and lumefantrin Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Artemether and lumefantrin Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Artemether and lumefantrin Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Artemether and lumefantrin Clinical Studies in the drug label.
How Supplied
There is limited information regarding Artemether and lumefantrin How Supplied in the drug label.
Storage
There is limited information regarding Artemether and lumefantrin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Artemether and lumefantrin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Artemether and lumefantrin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Artemether and lumefantrin Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Artemether and lumefantrin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Artemether and lumefantrin Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Artemether and lumefantrin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.