Chlorthalidone: Difference between revisions
No edit summary |
m (Protected "Chlorthalidone": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (28 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag= | |authorTag=Gerald Chi<!--Overview--> | ||
Gerald Chi | |genericName=Chlorthalidone | ||
|aOrAn=a | |||
<!--Overview--> | |drugClass=[[thiazide|thiazide-like]] [[diuretic]] | ||
|indicationType=treatment | |||
|genericName= | |indication=[[hypertension]] and [[edema]] associated with [[congestive heart failure]], [[hepatic cirrhosis]], and [[corticosteroid]] and [[estrogen]] therapy | ||
Chlorthalidone | |adverseReactions=[[dizziness]], [[lightheadedness]], and [[hyperuricemia]] | ||
|aOrAn= | |||
a | |||
|drugClass= | |||
[[thiazide|thiazide-like]] [[diuretic]] | |||
|indication= | |||
[[hypertension]] and [[edema]] associated with [[congestive heart failure]], [[hepatic cirrhosis]], and [[corticosteroid]] and [[estrogen]] therapy | |||
|adverseReactions= | |||
[[dizziness]], [[lightheadedness]], and [[hyperuricemia]] | |||
<!--Black Box Warning--> | <!--Black Box Warning--> | ||
|blackBoxWarningTitle=Title | |||
|blackBoxWarningTitle= | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i>Content | ||
Title | |||
|blackBoxWarningBody= | |||
<i><span style="color:#FF0000;">ConditionName: </span></i>Content | |||
<!--Adult Indications and Dosage--> | <!--Adult Indications and Dosage--> | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult=* Therapy should be initiated with the lowest possible dose, then titrated according to individual patient response. A single dose given in the morning with food is recommended; divided doses are unnecessary. | |||
|fdaLIADAdult= | |||
* Therapy should be initiated with the lowest possible dose, then titrated according to individual patient response. A single dose given in the morning with food is recommended; divided doses are unnecessary. | |||
===== Hypertension ===== | ===== Hypertension ===== | ||
| Line 59: | Line 37: | ||
<!--Guideline-Supported Use (Adult)--> | <!--Guideline-Supported Use (Adult)--> | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
|offLabelAdultGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | <!--Non–Guideline-Supported Use (Adult)--> | ||
|offLabelAdultNoGuideSupport======Hypertension as in Stroke Prophylaxis===== | |||
|offLabelAdultNoGuideSupport= | |||
=====Hypertension as in Stroke Prophylaxis===== | |||
* Dosing Information | * Dosing Information | ||
| Line 87: | Line 59: | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed=Safety and effectiveness in children have not been established. | |||
|fdaLIADPed= | |||
Safety and effectiveness in children have not been established. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | <!--Off-Label Use and Dosage (Pediatric)--> | ||
<!--Guideline-Supported Use (Pediatric)--> | <!--Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|offLabelPedGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | <!--Non–Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|offLabelPedNoGuideSupport= | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications=* [[Anuria]] | |||
|contraindications= | |||
* [[Anuria]] | |||
* [[Hypersensitivity]] to chlorthalidone or other [[sulfonamide]]-derived drugs | * [[Hypersensitivity]] to chlorthalidone or other [[sulfonamide]]-derived drugs | ||
<!--Warnings--> | <!--Warnings--> | ||
|warnings=* Thalitone® (chlorthalidone USP) should be used with caution in severe renal disease. In patients with renal disease, chlorthalidone or related drugs may precipitate azotemia. Cumulative effects of the drug may develop in patients with impaired renal function. | |||
|warnings= | |||
* Thalitone® (chlorthalidone USP) should be used with caution in severe renal disease. In patients with renal disease, chlorthalidone or related drugs may precipitate azotemia. Cumulative effects of the drug may develop in patients with impaired renal function. | |||
* Chlorthalidone should be used with caution in patients with impaired hepatic function or progressive liver disease, because minor alterations of fluid and electrolyte balance may precipitate hepatic coma. | * Chlorthalidone should be used with caution in patients with impaired hepatic function or progressive liver disease, because minor alterations of fluid and electrolyte balance may precipitate hepatic coma. | ||
| Line 147: | Line 104: | ||
<!--Clinical Trials Experience--> | <!--Clinical Trials Experience--> | ||
|clinicalTrials=There is limited information regarding <i>Clinical Trial Experience</i> of {{PAGENAME}} in the drug label. | |||
|clinicalTrials= | |||
There is limited information regarding <i>Clinical Trial Experience</i> of {{PAGENAME}} in the drug label. | |||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
|postmarketing=* Whenever adverse reactions are moderate or severe, chlorthalidone dosage should be reduced or therapy withdrawn. | |||
|postmarketing= | |||
* Whenever adverse reactions are moderate or severe, chlorthalidone dosage should be reduced or therapy withdrawn. | |||
* The following adverse reactions have been observed, but there is not enough systematic collection of data to support an estimate of their frequency. | * The following adverse reactions have been observed, but there is not enough systematic collection of data to support an estimate of their frequency. | ||
| Line 170: | Line 121: | ||
======Gastrointestinal====== | ======Gastrointestinal====== | ||
[[Anorexia]], gastric irritation, [[nausea]], [[vomiting]], [[cramping]], [[diarrhea]], [[constipation]], [[jaundice]] (intrahepatic cholestatic jaundice), [[pancreatitis]] | [[Anorexia]], gastric irritation, [[nausea]], [[vomiting]], [[cramping]], [[diarrhea]], [[constipation]], [[jaundice]] (intrahepatic cholestatic jaundice), and [[pancreatitis]] | ||
======Hematologic Reactions====== | ======Hematologic Reactions====== | ||
Leukopenia, agranulocytosis, thrombocytopenia, aplastic anemia | [[Leukopenia]], [[agranulocytosis]], [[thrombocytopenia]], and [[aplastic anemia]] | ||
======Hypersensitivity====== | ======Hypersensitivity====== | ||
Purpura, photosensitivity, rash, urticaria, necrotizing angiitis (cutaneous vasculitis), [[Lyell's syndrome]] ([[toxic epidermal necrolysis]]) | [[Purpura]], [[photosensitivity]], [[rash]], [[urticaria]], [[Systemic vasculitis|necrotizing angiitis]] ([[Systemic vasculitis|cutaneous vasculitis]]), and [[Lyell's syndrome]] ([[toxic epidermal necrolysis]]) | ||
======Miscellaneous====== | ======Miscellaneous====== | ||
Hyperglycemia, glycosuria, hyperuricemia, muscle spasm, weakness, restlessness, impotence | [[Hyperglycemia]], [[glycosuria]], [[hyperuricemia]], [[muscle spasm]], [[weakness]], [[restlessness]], and [[impotence]] | ||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
|drugInteractions=* Chlorthalidone may add to or potentiate the action of other [[antihypertensive]] drugs. | |||
* [[Insulin]] requirements in [[diabetic]] patients may be increased, decreased or unchanged. Higher dosage of [[oral hypoglycemic agent]]s may be required. | |||
* Chlorthalidone and related drugs may increase the responsiveness to [[tubocurarine]]. | |||
* Chlorthalidone and related drugs may decrease arterial responsiveness to [[norepinephrine]]. This diminution is not sufficient to preclude effectiveness of the pressor agent for therapeutic use. | |||
* [[Lithium]] renal clearance is reduced by chlorthalidone, increasing the risk of [[lithium]] toxicity. | |||
=====Drug/Laboratory Test Interactions===== | |||
* | * Chlorthalidone and related drugs may decrease serum PBI levels without signs of [[thyroid]] disturbance. | ||
<!--Use in Specific Populations--> | <!--Use in Specific Populations--> | ||
|useInPregnancyFDA=* '''Pregnancy Category B''' | |||
|useInPregnancyFDA= | :* Pregnancy/Teratogenic Effects | ||
* '''Pregnancy Category B''' | ::* Reproduction studies have been performed in the rat and the rabbit at doses up to 420 times the human dose and have revealed no evidence of harm to the fetus due to chlorthalidone. There are, however, no adequate and well-controlled studies in [[pregnant]] women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. | ||
:* Pregnancy/Non-Teratogenic Effects | |||
|useInPregnancyAUS= | ::* [[Thiazides]] cross the placental barrier and appear in cord blood. The use of chlorthalidone and related drugs in pregnant women requires that the anticipated benefits of the drug be weighed against possible hazards to the fetus. These hazards include fetal or neonatal [[jaundice]], [[thrombocytopenia]], and possibly other adverse reactions that have occurred in the adult. | ||
* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category C''' | :* The routine use of [[diuretics]] in an otherwise healthy woman is inappropriate and exposes mother and fetus to unnecessary hazard. Diuretics do not prevent development of toxemia of pregnancy and there is no satisfactory evidence that they are useful in the treatment of developed toxemia. | ||
:* [[Edema]] during [[pregnancy]] may arise from pathological causes or from the physiologic and mechanical consequences of [[pregnancy]]. Chlorthalidone is indicated in [[pregnancy]] when [[edema]] is due to pathologic causes just as it is in the absence of pregnancy. Dependent [[edema]] in pregnancy resulting from restriction of venous return by the expanded [[uterus]] is properly treated through elevation of the lower extremities and use of support hose; use of diuretics to lower intravascular volume in this case is illogical and unnecessary. There is hypervolemia during normal pregnancy that is harmful to neither the fetus nor the mother (in the absence of cardiovascular disease) but that is associated with [[edema]], including generalized [[edema]], in the majority of pregnant women. If this [[edema]] produces discomfort, increased recumbency will often provide relief. In rare instances, this edema may cause extreme discomfort that is not relieved by rest. In these cases, a short course of diuretics may provide relief and may be appropriate. | |||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category C''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing=* [[Thiazides]] are excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from chlorthalidone, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. | |||
|useInPed=* Safety and effectiveness in children have not been established. | |||
|useInGeri=* Clinical studies of chlorthalidone did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy. | |||
* This drug is known to be substantially excreted by the [[kidney]], and the risk of toxic reactions to this drug may be greater in patients with [[renal insufficiency|impaired renal function]]. Because elderly patients are more likely to have decreased [[renal function]], care should be taken in dose selection, and it may be useful to monitor [[renal function]]. | |||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
|useInGender= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential= | |||
There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp= | |||
There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* Oral | |||
|monitoring======Renal Function===== | |||
* This drug is known to be substantially excreted by the [[kidney]], and the risk of toxic reactions to this drug may be greater in patients with [[renal insufficiency|impaired renal function]]. Because elderly patients are more likely to have decreased [[renal function]], care should be taken in dose selection, and it may be useful to monitor [[renal function]]. | |||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
|IVCompat= | |||
There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose====Acute Overdose=== | |||
|overdose= | |||
===Acute Overdose=== | |||
====Signs and Symptoms==== | ====Signs and Symptoms==== | ||
* Symptoms of acute overdosage include [[nausea]], [[weakness]], [[dizziness]] and disturbances of [[electrolyte]] balance. | |||
* The oral LD50 of the drug in the mouse and the rat is more than 25,000 mg/kg body weight. | |||
* The minimum lethal dose (MLD) in humans has not been established. | |||
====Management==== | ====Management==== | ||
* There is no specific antidote but [[gastric lavage]] is recommended, followed by supportive treatment. | |||
* Where necessary, this may include [[intravenous]] [[dextrose]]-[[saline]] with [[potassium]], administered with caution. | |||
===Chronic Overdose=== | ===Chronic Overdose=== | ||
| Line 282: | Line 200: | ||
<!--Drugbox2--> | <!--Drugbox2--> | ||
|drugBox={{Drugbox2 | |||
|drugBox= | |||
{{Drugbox2 | |||
| verifiedrevid = 460034023 | | verifiedrevid = 460034023 | ||
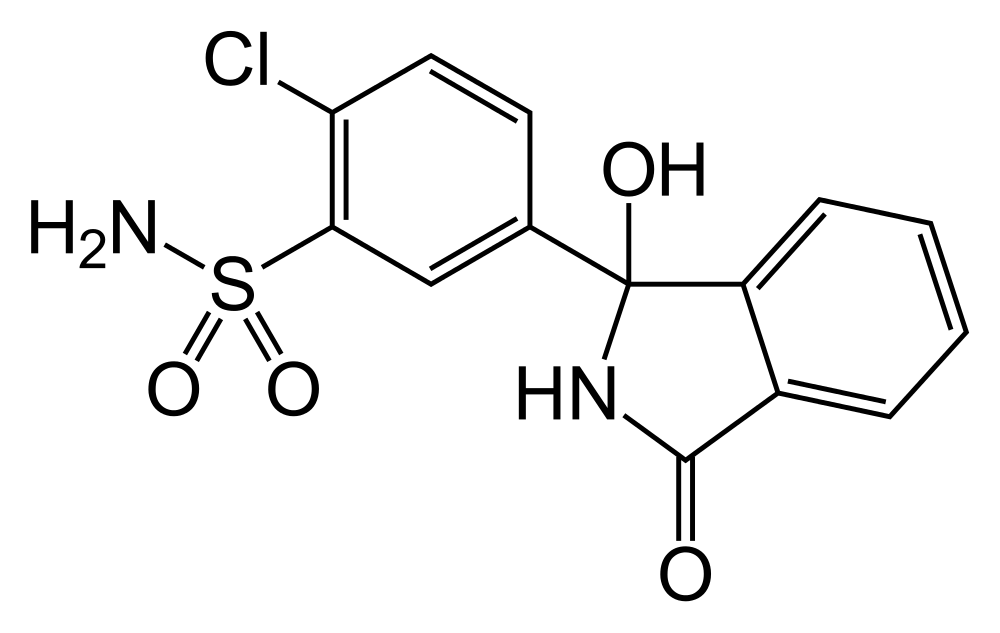
| IUPAC_name = (''RS'')-2-Chloro-5-(1-hydroxy-3-oxo-2,3-dihydro-1''H''-isoindol-1-yl)benzene-1-sulfonamide | | IUPAC_name = (''RS'')-2-Chloro-5-(1-hydroxy-3-oxo-2,3-dihydro-1''H''-isoindol-1-yl)benzene-1-sulfonamide | ||
| Line 339: | Line 254: | ||
| StdInChIKey = JIVPVXMEBJLZRO-UHFFFAOYSA-N | | StdInChIKey = JIVPVXMEBJLZRO-UHFFFAOYSA-N | ||
}} | }} | ||
<!--Mechanism of Action--> | <!--Mechanism of Action--> | ||
|mechAction=* Chlorthalidone is a long-acting oral [[diuretic]] with [[antihypertensive]] activity. Its diuretic action commences a mean of 2.6 hours after dosing and continues for up to 72 hours. The drug produces diuresis with increased excretion of [[sodium]] and [[chloride]]. The [[diuretic]] effects of chlorthalidone and the benzothiadiazine ([[thiazide]]) [[diuretics]] appear to arise from similar mechanisms and the maximal effect of chlorthalidone and the thiazides appear to be similar. The site of the action appears to be the [[distal convoluted tubule]] of the [[nephron]]. The [[diuretic]] effects of chlorthalidone lead to decreased extracellular fluid volume, plasma volume, [[cardiac output]], total exchangeable sodium, [[glomerular filtration rate]], and renal plasma flow. | |||
* Although the mechanism of action of chlorthalidone and related drugs is not wholly clear, [[sodium]] and water depletion appear to provide a basis for its [[antihypertensive]] effect. Like the [[thiazide]] [[diuretics]], chlorthalidone produces dose-related reductions in serum [[potassium]] levels, elevations in serum [[uric acid]] and [[blood glucose]], and it can lead to decreased [[sodium]] and [[chloride]] levels. | |||
<!--Structure--> | <!--Structure--> | ||
|structure=* Thalitone® (chlorthalidone USP) is an antihypertensive/diuretic supplied as 15 mg tablets for oral use. It is a monosulfamyl diuretic that differs chemically from thiazide diuretics in that a double ring system is incorporated in its structure. It is a racemic mixture of 2-chloro-5-(1-hydroxy-3-oxo-1-isoindolinyl) benzenesulfonamide, with the following structural formula: | |||
| | [[File:Chlorthalidone06.jpeg|400px|thumb|none|This image is provided by the National Library of Medicine.]] | ||
* Chlorthalidone is practically insoluble in water, in ether and in chloroform; soluble in methanol; slightly soluble in alcohol. | |||
* The inactive ingredients are colloidal silicon dioxide, lactose, magnesium stearate, microcrystalline cellulose, povidone, sodium starch glycolate. | |||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
|PD=* The mean plasma [[half-life]] of chlorthalidone is about 40 to 60 hours. It is eliminated primarily as unchanged drug in the urine. Non-renal routes of elimination have yet to be clarified. In the blood, approximately 75% of the drug is bound to plasma proteins. | |||
|PD= | |||
<!--Pharmacokinetics--> | <!--Pharmacokinetics--> | ||
|PK=* Thalitone® (chlorthalidone USP) has been formulated with PVP (povidone polyvinylpyrrolidone), a bioavailability enhancer that provides 104% to 116% [[bioavailability]] relative to an oral solution of chlorthalidone. Thalitone® cannot be substituted for other formulations of chlorthalidone and likewise, other formulations of chlorthalidone cannot be substituted for Thalitone®. | |||
|PK= | |||
<!--Nonclinical Toxicology--> | <!--Nonclinical Toxicology--> | ||
|nonClinToxic=There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | |||
|nonClinToxic= | |||
There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | |||
<!--Clinical Studies--> | <!--Clinical Studies--> | ||
|clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
|clinicalStudies= | |||
There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied=* White, kidney-shaped, compressed tablets coded M/024 containing 15 mg of chlorthalidone in bottles of 100 (NDC 61570-024-01). | |||
|howSupplied= | |||
* White, kidney-shaped, compressed tablets coded M/024 containing 15 mg of chlorthalidone in bottles of 100 (NDC 61570-024-01). | |||
* Storage: Store below 30°C (86°F). | * Storage: Store below 30°C (86°F). | ||
<!--Patient Counseling Information--> | <!--Patient Counseling Information--> | ||
|fdaPatientInfo=* Patients should inform their doctor if they have: | |||
:* [[Allergic reaction]] to chlorthalidone or other [[diuretics]] | |||
:* [[Asthma]] | |||
:* [[Kidney]] disease | |||
:* [[Liver]] disease | |||
:* [[Gout]] | |||
:* [[Systemic lupus erythematosus]] | |||
:* Taking other drugs such as [[cortisone]], [[digitalis]], [[lithium]] carbonate, or drugs for [[diabetes]] | |||
* Patients should be cautioned to contact their physician if they experience any of the following symptoms of [[potassium]] loss: excess [[thirst]], [[tiredness]], [[drowsiness]], [[restlessness]], [[muscle pain]]s or [[cramp]]s, [[nausea]], [[vomiting]] or increased [[heart rate]] or [[pulse]]. | |||
* Patients should be cautioned to contact their physician if they experience any of the following symptoms of potassium loss: excess thirst, tiredness, drowsiness, restlessness, muscle | |||
* Patients should also be cautioned that taking alcohol can increase the chance of dizziness occurring. | * Patients should also be cautioned that taking alcohol can increase the chance of [[dizziness]] occurring. | ||
<!--Precautions with Alcohol--> | <!--Precautions with Alcohol--> | ||
|alcohol=[[Orthostatic hypotension]] may occur and may be aggravated by [[alcohol]]. Patients should be cautioned that taking [[alcohol]] can increase the chance of [[dizziness]] occurring. | |||
| | <!--Brand Names--> | ||
|brandNames=Thalitone®<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = THALITONE (CHLORTHALIDONE) TABLET [MONARCH PHARMACEUTICALS, INC] | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=e2eb7dad-3ea3-439c-dcbb-d1d61aa49dfc | publisher = | date = | accessdate = 30 June 2014 }}</ref> | |||
<!--Look-Alike Drug Names--> | |||
|lookAlike=N/A<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | |||
<!-- | <!--Drug Shortage Status--> | ||
|drugShortage= | |||
}} | |||
{{PillImage | |||
|fileName=Chlorthalidone07.jpeg | |||
|drugName=Thalitone 15 MG Oral Tablet | |||
|NDC=61570-024 | |||
|drugAuthor=Monarch Pharmaceuticals, Inc | |||
|ingredients=Chlorthalidone | |||
|pillImprint=M;024 | |||
|dosageValue=15 | |||
|dosageUnit=mg | |||
|pillColor=White | |||
|pillShape=FreeForm | |||
|pillSize=8 | |||
|pillScore=1 | |||
}} | |||
<!--Pill Image--> | |||
<!--Label Display Image--> | |||
{{LabelImage | |||
|fileName=Chlorthalidone01.jpeg|This image is provided by the National Library of Medicine. | |||
}} | |||
| | {{LabelImage | ||
|fileName=Chlorthalidone02.jpeg|This image is provided by the National Library of Medicine. | |||
}} | }} | ||
{{LabelImage | |||
|fileName=Chlorthalidone03.jpeg|This image is provided by the National Library of Medicine. | |||
}} | |||
{{ | {{LabelImage | ||
|fileName= | |fileName=Chlorthalidone04.jpeg|This image is provided by the National Library of Medicine. | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName= | |fileName=Chlorthalidone05.jpeg|This image is provided by the National Library of Medicine. | ||
}} | }} | ||
<!--Category--> | <!--Category--> | ||
[[Category:Diuretics]] | |||
[[Category:Sulfonamides]] | |||
[[Category:Cardiovascular Drugs]] | [[Category:Cardiovascular Drugs]] | ||
[[Category:Drug]] | [[Category:Drug]] | ||
Latest revision as of 19:02, 18 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Chlorthalidone is a thiazide-like diuretic that is FDA approved for the treatment of hypertension and edema associated with congestive heart failure, hepatic cirrhosis, and corticosteroid and estrogen therapy. Common adverse reactions include dizziness, lightheadedness, and hyperuricemia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Therapy should be initiated with the lowest possible dose, then titrated according to individual patient response. A single dose given in the morning with food is recommended; divided doses are unnecessary.
Hypertension
- Dosing Information
- Initial dose: 15 mg PO qd
- If the response is insufficient after a suitable trial, the dosage may be increased to 30 mg and then to a single daily dose of 45–50 mg.
- If additional control is required, the addition of a second antihypertensive drug is recommended.
- Increases in serum uric acid and decreases in serum potassium are dose-related over the 15–50 mg/day range and beyond.
Edema
- Dosing Information
- Initial dose: Adults, initially 30–60 mg PO qd or 60 mg PO qod.
- Some patients may require 90–120 mg at these intervals or up to 120 mg daily. Dosages above this level, however, do not usually produce a greater response.
- Maintenance dose: maintenance doses may often be lower than initial doses and should be adjusted according to the individual patient.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Chlorthalidone in adult patients.
Non–Guideline-Supported Use
Hypertension as in Stroke Prophylaxis
- Dosing Information
- 12.5–25 mg PO qd[1]
Left Ventricular Hypertrophy
- Dosing Information
- 100 mg PO qd[2]
Ménière's Disease
- Dosing Information
- 50–200 mg PO qd[3]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness in children have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Chlorthalidone in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Chlorthalidone in pediatric patients.
Contraindications
- Anuria
- Hypersensitivity to chlorthalidone or other sulfonamide-derived drugs
Warnings
- Thalitone® (chlorthalidone USP) should be used with caution in severe renal disease. In patients with renal disease, chlorthalidone or related drugs may precipitate azotemia. Cumulative effects of the drug may develop in patients with impaired renal function.
- Chlorthalidone should be used with caution in patients with impaired hepatic function or progressive liver disease, because minor alterations of fluid and electrolyte balance may precipitate hepatic coma.
- Sensitivity reactions may occur in patients with a history of allergy or bronchial asthma.
- The possibility of exacerbation or activation of systemic lupus erythematosus has been reported with thiazide diuretics which are structurally related to chlorthalidone. However, systemic lupus erythematosus has not been reported following chlorthalidone administration.
Precautions
- General
- Hypokalemia and other electrolyte abnormalities, including hyponatremia and hypochloremic alkalosis, are common in patients receiving chlorthalidone. These abnormalities are dose-related but may occur even at the lowest marketed doses of chlorthalidone. Serum electrolytes should be determined before initiating therapy and at periodic intervals during therapy. Serum and urine electrolyte determinations are particularly important when the patient is vomiting excessively or receiving parenteral fluids. All patients taking chlorthalidone should be observed for clinical signs of electrolyte imbalance, including dryness of mouth, thirst, weakness, lethargy, drowsiness, restlessness, muscle pains or cramps, muscular fatigue, hypotension, oliguria, tachycardia, palpitations, and gastrointestinal disturbances, such as nausea and vomiting.
- Digitalis therapy may exaggerate metabolic effects of hypokalemia especially with reference to myocardial activity.
- Any chloride deficit is generally mild and usually does not require specific treatment except under extraordinary circumstances (as in liver disease or renal disease). Dilutional hyponatremia may occur in edematous patients in hot weather; appropriate therapy is water restriction, rather than administration of salt, except in rare instances when the hyponatremia is life-threatening. In cases of actual salt depletion, appropriate replacement is the therapy of choice.
- Thiazide-like diuretics have been shown to increase the urinary excretion of magnesium; this may result in hypomagnesemia.
- Calcium excretion is decreased by thiazide-like drugs. Pathological changes in the parathyroid gland with hypercalcemia and hypophosphatemia have been observed in a few patients on thiazide therapy. The common complications of hyperparathyroidism such as renal lithiasis, bone resorption and peptic ulceration have not been seen.
- Hyperuricemia may occur or frank gout may be precipitated in certain patients receiving chlorthalidone.
- Other
- Increases in serum glucose may occur and latent diabetes mellitus may become manifest during chlorthalidone therapy. Chlorthalidone and related drugs may decrease serum protein-bound iodine (PBI) levels without signs of thyroid disturbance.
- Laboratory Tests
- Periodic determination of serum electrolytes to detect possible electrolyte imbalance should be performed at appropriate intervals.
- All patients receiving chlorthalidone should be observed for clinical signs of fluid or electrolyte imbalance: namely, hyponatremia, hypochloremic alkalosis and hypokalemia. Serum and urine electrolyte determinations are particularly important when the patient is vomiting excessively or receiving parenteral fluids.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Chlorthalidone in the drug label.
Postmarketing Experience
- Whenever adverse reactions are moderate or severe, chlorthalidone dosage should be reduced or therapy withdrawn.
- The following adverse reactions have been observed, but there is not enough systematic collection of data to support an estimate of their frequency.
Central Nervous System
Dizziness, vertigo, paresthesias, headache, and xanthopsia
Cardiovascular
Orthostatic hypotension may occur and may be aggravated by alcohol, barbiturates or narcotics.
Gastrointestinal
Anorexia, gastric irritation, nausea, vomiting, cramping, diarrhea, constipation, jaundice (intrahepatic cholestatic jaundice), and pancreatitis
Hematologic Reactions
Leukopenia, agranulocytosis, thrombocytopenia, and aplastic anemia
Hypersensitivity
Purpura, photosensitivity, rash, urticaria, necrotizing angiitis (cutaneous vasculitis), and Lyell's syndrome (toxic epidermal necrolysis)
Miscellaneous
Hyperglycemia, glycosuria, hyperuricemia, muscle spasm, weakness, restlessness, and impotence
Drug Interactions
- Chlorthalidone may add to or potentiate the action of other antihypertensive drugs.
- Insulin requirements in diabetic patients may be increased, decreased or unchanged. Higher dosage of oral hypoglycemic agents may be required.
- Chlorthalidone and related drugs may increase the responsiveness to tubocurarine.
- Chlorthalidone and related drugs may decrease arterial responsiveness to norepinephrine. This diminution is not sufficient to preclude effectiveness of the pressor agent for therapeutic use.
- Lithium renal clearance is reduced by chlorthalidone, increasing the risk of lithium toxicity.
Drug/Laboratory Test Interactions
- Chlorthalidone and related drugs may decrease serum PBI levels without signs of thyroid disturbance.
Use in Specific Populations
Pregnancy
- Pregnancy Category B
- Pregnancy/Teratogenic Effects
- Reproduction studies have been performed in the rat and the rabbit at doses up to 420 times the human dose and have revealed no evidence of harm to the fetus due to chlorthalidone. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- Pregnancy/Non-Teratogenic Effects
- Thiazides cross the placental barrier and appear in cord blood. The use of chlorthalidone and related drugs in pregnant women requires that the anticipated benefits of the drug be weighed against possible hazards to the fetus. These hazards include fetal or neonatal jaundice, thrombocytopenia, and possibly other adverse reactions that have occurred in the adult.
- The routine use of diuretics in an otherwise healthy woman is inappropriate and exposes mother and fetus to unnecessary hazard. Diuretics do not prevent development of toxemia of pregnancy and there is no satisfactory evidence that they are useful in the treatment of developed toxemia.
- Edema during pregnancy may arise from pathological causes or from the physiologic and mechanical consequences of pregnancy. Chlorthalidone is indicated in pregnancy when edema is due to pathologic causes just as it is in the absence of pregnancy. Dependent edema in pregnancy resulting from restriction of venous return by the expanded uterus is properly treated through elevation of the lower extremities and use of support hose; use of diuretics to lower intravascular volume in this case is illogical and unnecessary. There is hypervolemia during normal pregnancy that is harmful to neither the fetus nor the mother (in the absence of cardiovascular disease) but that is associated with edema, including generalized edema, in the majority of pregnant women. If this edema produces discomfort, increased recumbency will often provide relief. In rare instances, this edema may cause extreme discomfort that is not relieved by rest. In these cases, a short course of diuretics may provide relief and may be appropriate.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category C
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Chlorthalidone in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Chlorthalidone during labor and delivery.
Nursing Mothers
- Thiazides are excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from chlorthalidone, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in children have not been established.
Geriatic Use
- Clinical studies of chlorthalidone did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
- This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Gender
There is no FDA guidance on the use of Chlorthalidone with respect to specific gender populations.
Race
There is no FDA guidance on the use of Chlorthalidone with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Chlorthalidone in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Chlorthalidone in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Chlorthalidone in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Chlorthalidone in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
Renal Function
- This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
IV Compatibility
There is limited information regarding IV Compatibility of Chlorthalidone in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Symptoms of acute overdosage include nausea, weakness, dizziness and disturbances of electrolyte balance.
- The oral LD50 of the drug in the mouse and the rat is more than 25,000 mg/kg body weight.
- The minimum lethal dose (MLD) in humans has not been established.
Management
- There is no specific antidote but gastric lavage is recommended, followed by supportive treatment.
- Where necessary, this may include intravenous dextrose-saline with potassium, administered with caution.
Chronic Overdose
There is limited information regarding Chronic Overdose of Chlorthalidone in the drug label.
Pharmacology
| |
| |
1 : 1 mixture (racemate)Chlortalidone
| |
| Systematic (IUPAC) name | |
| (RS)-2-Chloro-5-(1-hydroxy-3-oxo-2,3-dihydro-1H-isoindol-1-yl)benzene-1-sulfonamide | |
| Identifiers | |
| CAS number | |
| ATC code | C03 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 338.766 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 75% |
| Metabolism | ? |
| Half life | 40 hours |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status | |
| Routes | Oral |
Mechanism of Action
- Chlorthalidone is a long-acting oral diuretic with antihypertensive activity. Its diuretic action commences a mean of 2.6 hours after dosing and continues for up to 72 hours. The drug produces diuresis with increased excretion of sodium and chloride. The diuretic effects of chlorthalidone and the benzothiadiazine (thiazide) diuretics appear to arise from similar mechanisms and the maximal effect of chlorthalidone and the thiazides appear to be similar. The site of the action appears to be the distal convoluted tubule of the nephron. The diuretic effects of chlorthalidone lead to decreased extracellular fluid volume, plasma volume, cardiac output, total exchangeable sodium, glomerular filtration rate, and renal plasma flow.
- Although the mechanism of action of chlorthalidone and related drugs is not wholly clear, sodium and water depletion appear to provide a basis for its antihypertensive effect. Like the thiazide diuretics, chlorthalidone produces dose-related reductions in serum potassium levels, elevations in serum uric acid and blood glucose, and it can lead to decreased sodium and chloride levels.
Structure
- Thalitone® (chlorthalidone USP) is an antihypertensive/diuretic supplied as 15 mg tablets for oral use. It is a monosulfamyl diuretic that differs chemically from thiazide diuretics in that a double ring system is incorporated in its structure. It is a racemic mixture of 2-chloro-5-(1-hydroxy-3-oxo-1-isoindolinyl) benzenesulfonamide, with the following structural formula:
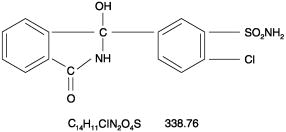
- Chlorthalidone is practically insoluble in water, in ether and in chloroform; soluble in methanol; slightly soluble in alcohol.
- The inactive ingredients are colloidal silicon dioxide, lactose, magnesium stearate, microcrystalline cellulose, povidone, sodium starch glycolate.
Pharmacodynamics
- The mean plasma half-life of chlorthalidone is about 40 to 60 hours. It is eliminated primarily as unchanged drug in the urine. Non-renal routes of elimination have yet to be clarified. In the blood, approximately 75% of the drug is bound to plasma proteins.
Pharmacokinetics
- Thalitone® (chlorthalidone USP) has been formulated with PVP (povidone polyvinylpyrrolidone), a bioavailability enhancer that provides 104% to 116% bioavailability relative to an oral solution of chlorthalidone. Thalitone® cannot be substituted for other formulations of chlorthalidone and likewise, other formulations of chlorthalidone cannot be substituted for Thalitone®.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Chlorthalidone in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Chlorthalidone in the drug label.
How Supplied
- White, kidney-shaped, compressed tablets coded M/024 containing 15 mg of chlorthalidone in bottles of 100 (NDC 61570-024-01).
- Storage: Store below 30°C (86°F).
Storage
There is limited information regarding Chlorthalidone Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Chlorthalidone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Chlorthalidone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should inform their doctor if they have:
- Patients should be cautioned to contact their physician if they experience any of the following symptoms of potassium loss: excess thirst, tiredness, drowsiness, restlessness, muscle pains or cramps, nausea, vomiting or increased heart rate or pulse.
- Patients should also be cautioned that taking alcohol can increase the chance of dizziness occurring.
Precautions with Alcohol
Orthostatic hypotension may occur and may be aggravated by alcohol. Patients should be cautioned that taking alcohol can increase the chance of dizziness occurring.
Brand Names
Thalitone®[4]
Look-Alike Drug Names
N/A[5]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Perry, HM.; Davis, BR.; Price, TR.; Applegate, WB.; Fields, WS.; Guralnik, JM.; Kuller, L.; Pressel, S.; Stamler, J. (2000). "Effect of treating isolated systolic hypertension on the risk of developing various types and subtypes of stroke: the Systolic Hypertension in the Elderly Program (SHEP)". JAMA. 284 (4): 465–71. PMID 10904510. Unknown parameter
|month=ignored (help) - ↑ Cherchi, A.; Sau, F.; Seguro, C. (1987). "Possible regression of left ventricular hypertrophy during antihypertensive treatment with diuretics and/or beta blockers". J Clin Hypertens. 3 (2): 216–25. PMID 2886561. Unknown parameter
|month=ignored (help) - ↑ Klockhoff, I.; Lindblom, U.; Stahle, J. (1974). "Diuretic treatment of Meniere disease. Long-term results with chlorthalidone". Arch Otolaryngol. 100 (4): 262–5. PMID 4412853. Unknown parameter
|month=ignored (help) - ↑ "THALITONE (CHLORTHALIDONE) TABLET [MONARCH PHARMACEUTICALS, INC]". Retrieved 30 June 2014.
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Chlorthalidone |Pill Name=Chlorthalidone07.jpeg |Drug Name=Thalitone 15 MG Oral Tablet |Pill Ingred=Chlorthalidone|+sep=; |Pill Imprint=M;024 |Pill Dosage=15 mg |Pill Color=White|+sep=; |Pill Shape=FreeForm |Pill Size (mm)=8 |Pill Scoring=1 |Pill Image= |Drug Author=Monarch Pharmaceuticals, Inc |NDC=61570-024
}}
{{#subobject:
|Label Page=Chlorthalidone |Label Name=Chlorthalidone01.jpeg
}}
{{#subobject:
|Label Page=Chlorthalidone |Label Name=Chlorthalidone02.jpeg
}}
{{#subobject:
|Label Page=Chlorthalidone |Label Name=Chlorthalidone03.jpeg
}}
{{#subobject:
|Label Page=Chlorthalidone |Label Name=Chlorthalidone04.jpeg
}}
{{#subobject:
|Label Page=Chlorthalidone |Label Name=Chlorthalidone05.jpeg
}}