Ticarcillin clavulanate microbiology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mohamed Moubarak, M.D. [2]
Microbiology
- Mechanism of Action:
Ticarcillin disrupts bacterial cell wall development by inhibiting peptidoglycan synthesis and/or by interacting with penicillin‑binding proteins.
Ticarcillin is susceptible to degradation by β‑lactamases, so the spectrum of activity does not normally include organisms which produce these enzymes.
Clavulanic acid is a β‑lactam, structurally related to the penicillins, which inactivates some β‑lactamase enzymes commonly found in bacteria resistant to penicillins and cephalosporins. In particular, it has good activity against the clinically important plasmid‑mediated β‑lactamases frequently responsible for transferred drug resistance.
The formulation of ticarcillin with clavulanic acid in TIMENTIN protects ticarcillin from degradation by β‑lactamase enzymes, effectively extending the antibacterial spectrum of ticarcillin to include many bacteria normally resistant to ticarcillin and other β‑lactam antibacterials.
Interaction With Other Antimicrobials: In vitro synergism between TIMENTIN and gentamicin, tobramycin, or amikacin against multi-resistant isolates of Pseudomonas aeruginosa has been demonstrated.
Ticarcillin/clavulanic acid has been shown to be active against most isolates of the following bacteria, both in vitro and in clinical infections [see Indications and Usage (1)].
Gram-positive bacteria
Staphylococcus aureus (methicillin-susceptible isolates only)
Staphylococcus epidermidis (methicillin-susceptible isolates only)
Gram-negative bacteria'
Citrobacter species
Enterobacter species
Klebsiella species
Pseudomonas species
Anaerobic bacteria
Bacteroides fragilis group
Prevotella melaninogenicus
a β‑lactamase‑negative, ampicillin‑resistant (BLNAR) isolates of H. influenzae must be considered resistant to ticarcillin/clavulanic acid.
The following in vitro data are available, but their clinical significance is unknown.
At least 90 percent of the following bacteria exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for ticarcillin/clavulanic acid. However, the efficacy of ticarcillin/clavulanic acid in treating clinical infections due to these bacteria have not been established in adequate and well-controlled clinical trials.
Gram positive bacteria
Streptococcus agalactiae (Group B)
Streptococcus pneumoniae (penicillin-susceptible isolates only)
Viridans group streptococci
Gram negative bacteria
Anaerobic bacteria
Clostridium species
Eubacterium species
Fusobacterium species
F. nucleatum
F. necrophorum
Peptostreptococcus species
Veillonella species
- Susceptibility Testing:
When available, the clinical microbiology laboratory should provide the results of in vitro susceptibility test results for antimicrobial drug products used in local hospitals and practice areas to the physician as periodic reports that describe the susceptibility profile of nosocomial and community-acquired pathogens. These reports should aid the physician in selecting an antibacterial drug product for treatment.
- Dilution Techniques:
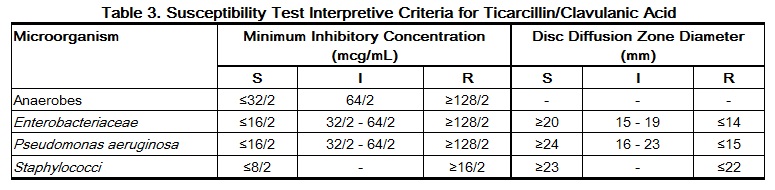
Quantitative methods are used to determine antimicrobial MICs. These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized test method2,4 (broth and/or agar). The MIC values should be interpreted according to criteria provided in Table 3.
- Diffusion Techniques:
Quantitative methods that require measurement of zone diameters can also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. The zone size provides an estimate of the susceptibility of bacteria to antimicrobial compounds. The zone size should be determined using a standardized test method.3,4 These procedures use paper disks impregnated with 85 mcg of ticarcillin/clavulanate potassium (75 mcg ticarcillin plus 10 mcg clavulanate potassium) to test the susceptibility of bacteria to ticarcillin/clavulanic acid. The disc diffusion interpretive criteria are provided in Table 3.
- Anaerobic Techniques:
For anaerobic bacteria, susceptibility to ticarcillin/clavulanic acid can be determined by standardized test methods.4,5 The MIC values obtained should be interpreted according to the criteria in Table 3.
A report of “Susceptible” indicates the antimicrobial is likely to inhibit growth of the pathogen if the antimicrobial compound reaches the concentrations at the infection site necessary to inhibit growth of the pathogen. A report of “Intermediate” indicates that the result should be considered equivocal, and, if the bacterium is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug product is physiologically concentrated or in situations where a high dosage of the drug product can be used. This category also provides a buffer zone that prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of “Resistant” indicates that the antimicrobial is not likely to inhibit growth of the pathogen if the antimicrobial compound reaches the concentrations usually achievable at the infection site; other therapy should be selected.
Quality Control: Standardized susceptibility test procedures require the use of laboratory controls to monitor and ensure the accuracy and precision of supplies and reagents used in the assay, and the techniques of the individual performing the tests.2,3,4,5 Standard ticarcillin/clavulanic acid powder should provide the following range of MIC values noted in Table 4. For the diffusion technique using the 85 mcg of ticarcillin/clavulanate potassium (75 mcg ticarcillin plus 10 mcg clavulanate potassium), the criteria in Table 4 should be achieved.[1]
References
Adapted from the FDA Package Insert.