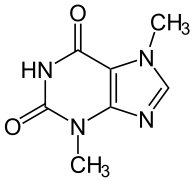
Theobromine
 | |
 | |
| Clinical data | |
|---|---|
| Synonyms | xantheose diurobromine 3,7-dimethylxanthine |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic demethylation and oxidation[1] |
| Elimination half-life | 7.1 +/- 0.7 hours |
| Excretion | Renal (10% unchanged, rest as metabolites)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C7H8N4O2[2] |
| Molar mass | 180.164 g/mol |
| 3D model (JSmol) | |
| |
|
WikiDoc Resources for Theobromine |
|
Articles |
|---|
|
Most recent articles on Theobromine Most cited articles on Theobromine |
|
Media |
|
Powerpoint slides on Theobromine |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Theobromine at Clinical Trials.gov Clinical Trials on Theobromine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Theobromine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Theobromine Discussion groups on Theobromine Patient Handouts on Theobromine Directions to Hospitals Treating Theobromine Risk calculators and risk factors for Theobromine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Theobromine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Theobromine, also known as xantheose,[2] is a bitter alkaloid of the cacao plant, and is therefore found in chocolate. It is in the methylxanthine class of chemical compounds,[3] which also includes the similar compounds theophylline and caffeine.[2] Despite its name, the compound contains no bromine — theobromine is derived from Theobroma, the name of the genus of the cacao tree, (which itself is made up of the Greek roots theo ("God") and broma ("food"), meaning "food of the gods")[4] with the suffix -ine given to alkaloids and other basic nitrogen-containing compounds.[5]
Theobromine is a water insoluble, crystalline, bitter powder; the colour has been listed as either white or colourless.[6] It has a similar, but lesser, effect to caffeine, making it a lesser homologue. Theobromine is an isomer of theophylline as well as paraxanthine. Theobromine is categorized as a dimethyl xanthine,[7] which means it is a xanthine[8] with two methyl groups.[9]
Theobromine was first isolated from the seeds of the cacao tree in 1878[10] and then shortly afterwards was synthesized from xanthine by Hermann Emil Fischer.[11]
Sources

Theobromine is the primary alkaloid found in cocoa and chocolate; chocolate contains 0.5-2.7% theobromine. Theobromine can also be found in small amounts in the kola nut (1.0-2.5%), the guarana berry, and the tea plant.[12]
Cocoa powder such as Hershey's cocoa contains 108mg of (or 2.16%) theobromine per tablespoon (5g) of powder.[13] However, cocoa powder with more concentrated amounts of theobromine up to at least 10% also exists.[14] Chocolate contains 0.5-2.7% theobromine, although white chocolate contains only trace amounts.[15] Theobromine can also be found in small amounts in the kola nut (1.0-2.5%), the guarana berry, and the tea plant.[12]
In the human liver, caffeine is metabolised by enzymes into 10% theobromine, 4% theophylline, and 80% paraxanthine.[16]
The plant species with the largest amounts of theobromine are:[17]
- Theobroma cacao
- Theobroma bicolor
- Yerba mate
- Camellia sinensis
- Cola acuminata
- Theobroma angustifolium
- Guarana
- Coffea arabica
The mean theobromine concentrations in cocoa and carob products are:[18]
| Item | Mean theobromine content (mg/g) |
|---|---|
| Cocoa | 20.3 |
| Cocoa cereals | 0.695 |
| Chocolate bakery products | 1.47 |
| Chocolate toppings | 1.95 |
| Cocoa beverages | 2.66 |
| Chocolate ice creams | 0.621 |
| Chocolate milks | 0.226 |
| Carob products | 0-0.504 |
Therapeutic uses
Following its discovery in the late 1800s, theobromine was put to use by 1916, where it was recommended by the publication Principles of Medical Treatment as a treatment for edema (excessive liquid in parts of the body), syphilitic angina attacks, and degenerative angina.[19] The American Journal of Clinical Nutrition notes that theobromine was once used as a treatment for other circulatory problems including arteriosclerosis, certain vascular diseases, angina pectoris, and hypertension.[20]
In modern medicine, theobromine is used as a vasodilator (a blood vessel widener), an aid in urinating, and heart stimulant.[2] In addition, the future use of theobromine in such fields as cancer prevention has been patented.[21]
Theobromine has also been used in birth defect experiments involving mice and rabbits. A decreased fetal weight was noted in rabbits following forced feeding, but not after other administration of theobromine. Birth defects were not seen in rats.[22]
Pharmacology
In the human liver, theobromine is metabolized into methylxanthine and subsequently into methyluric acid.[23]
As a methylated xanthine, theobromine is a potent Cyclic adenosine monophosphate (cAMP) phosphodiesterase inhibitor;[8] this means that it helps prevent the enzyme phosphodiesterase from converting the active cAMP to an inactive form.[24] Cyclic Adenosine Monophosphate works as a second messenger in many hormone- and neurotransmitter-controlled metabolic systems, such as the breakdown of glycogen. When the inactivation of cAMP is inhibited by a compound such as theobromine, the effects of the neurotransmitter or hormone which stimulated the production of cAMP are much longer lived. The net result is generally a stimulatory effect.[25]
Effects
Humans
While theobromine and caffeine are similar in that they are related alkaloids, theobromine has a lesser impact on the human central nervous system and it stimulates the heart to a greater degree.[26] While theobromine is not as addictive, it has been cited as possibly causing addiction to chocolate.[27] A Sexual Odyssey: From Forbidden Fruit to Cybersex discusses how chocolate's alleged aphrodisiac effects may be caused by a number of factors. These include the stimulative effects of theobromine, pleasure induced by the hypothalamus as an effect of chocolate's sweet and fatty nature, or how chocolate affects the levels of serotonin. While serotonin has a pleasurable effect, in high concentrations it can be converted to melatonin which in large amounts reduces sexual drive.[26]
As it is a myocardial stimulant as well as a vasodilator, it increases heartbeat, yet it also dilates blood vessels, causing a reduced blood pressure.[28] However, a recent paper published suggested that the decrease in blood pressure may be caused by flavanols.[20] Furthermore, its draining effect allows it to be used to treat cardiac failure, which can be caused by an excessive accumulation of fluid.[28]
A 2005 study published by Imperial College London concluded that theobromine has an antitussive (cough-reducing) effect superior to codeine by suppressing vagus nerve activity.[29] Additionally, theobromine is helpful in treating asthma since it relaxes the smooth muscles, including the ones found in the bronchi.[30]
There is a possible association between theobromine and an increased risk of suffering from prostate cancer.[31]
Theobromine can cause sleeplessness, tremors, restlessness, anxiety, as well as contribute to increased production of urine.[30] Additional side effects include loss of appetite, nausea, and vomiting.[32]
Animals
The amount of theobromine found in chocolate is small enough that it can be safely consumed by humans, but animals that metabolize theobromine more slowly, such as dogs, can succumb to theobromine poisoning from as little as 50 grams of chocolate for a smaller dog and 400 grams for an average-sized dog. Complications include digestive issues, dehydration, excitability, and a slow heart rate. Later stages of theobromine poisoning include epileptic-like seizures and death. If caught early on, theobromine poisoning is treatable.[33]
Other
Theobromine is known to induce gene mutations in lower eukaryotes and bacteria. At the time of a 1997 report by the IARC, genetic mutations had not been found in higher eukaryotes, such as humans.[34]
References
- ↑ 1.0 1.1 Template:Fr icon "Theobromine". BIAM. March 29, 2000.
- ↑ 2.0 2.1 2.2 2.3 William Marias Malisoff (1943). Dictionary of Bio-Chemistry and Related Subjects. Philosophical Library. pp. 311, 530, 573. ISBN B0006AQ0NU Check
|isbn=value: invalid character (help). - ↑ Baer, Donald M. (1997). Environment and Behavior. Westview Press. p. 200. Unknown parameter
|coauthors=ignored (help) - ↑ Bennett, Alan Weinberg (2002). The World of Caffeine: The Science and Culture of the World's Most Popular Drug. Routledge, New York. ISBN 0415927234. Unknown parameter
|coauthors=ignored (help) (note: the book incorrectly notes that the name "Theobroma" is derived from Latin) - ↑ "-ine."The American Heritage® Dictionary of the English Language, Fourth Edition. Houghton Mifflin Company. 2004. ISBN 0395711460.
- ↑ "theobromine". Dictionary.com. For convenience, the direct source of the three definitions used has been cited.
- ↑ "Theobromine". On-Line Medical Dictionary.
- ↑ 8.0 8.1 "Xanthine". On-Line Medical Dictionary.
- ↑ "Dimethyl". On-Line Medical Dictionary.
- ↑ Walter Sneader (2005). Drug Discovery: A History. John Wiley & Sons. ISBN 0471899801.
- ↑ Thomas Edward Thorpe (1902). Essays in Historical Chemistry. The MacMillan Company.
- ↑ 12.0 12.1 Sir Ghillean Prance, Mark Nesbitt (2004). The Cultural History of Plants. New York: Routledge. p. 137, 175, 178–180. ISBN 0415927463.
- ↑ "Theobromine content of Hershey's confectionery products". The Hershey Company. Retrieved 2008-04-07.
- ↑ "AmerMed cocoa extract with 10% theobromine". AmerMed. Retrieved 2008-04-13.
- ↑ America Is Going Sweet on White Chocolate - New York Times
- ↑ "Caffeine". The Pharmacogenetics and Pharmacogenomics Knowledge Base.
- ↑ "Activities of a Specific Chemical Query - Theobromine". United States Department of Agriculture. Retrieved 2007-02-23.
- ↑ Craig, Winston J. (1984). "Caffeine and theobromine levels in cocoa and carob products". Journal of Food Science. 49 (1): 302–303. doi:10.1111/j.1365-2621.1984.tb13737.x. Retrieved 2008-04-20.
Mean theobromine and caffeine levels respectively, were 0.695 mg/g and 0.071 mg/g in cocoa cereals; 1.47 mg/g and 0.152 mg/g in chocolate bakery products; 1.95 mg/g and 0.138 mg/g in chocolate toppings; 2.66 mg/g and 0.208 mg/g in cocoa beverages; 0.621 mg/g and 0.032 mg/g in chocolate ice creams; 0.226 mg/g and 0.011 mg/g in chocolate milks; 74.8 mg/serving and 6.5 mg/serving in chocolate puddings. Theobromine and caffeine levels in carob products ranged from 0-0.504 mg/g and 0-0.067 mg/g, respectively.
Unknown parameter|coauthors=ignored (help) - ↑ George Cheever Shattuck (1916). Principles of medical treatment. W.M. Leonard. p. 15, 39, 41.
- ↑ 20.0 20.1 Kelly, Caleb J (2005). "Effects of theobromine should be considered in future studies". American Journal of Clinical Nutrition. 82 (2): 486-8. Unknown parameter
|month=ignored (help) - ↑ US 6693104, "Theobromine with an anti-carcinogenic activity"
- ↑ Rambali B, Andel I van, Schenk E, Wolterink G, Werken G van de, Stevenson H, Vleeming W (2002). "[The contribution of cocoa additive to cigarette smoking addiction]" (PDF). RIVM (report 650270002/2002).- The National Institute for Public Health and the Environment (Netherlands)
- ↑ Template:Cite paper
- ↑ * "Phosphodiesterase". On-Line Medical Dictionary.
- "Inhibitor". On-Line Medical Dictionary.
- ↑ David L. Nelson, Michael M. Cox (2005). Lehninger Principles of Biochemistry. W.H. Freeman and Company. pp. 435–439. ISBN 0716743396.
- ↑ 26.0 26.1 Kenneth Maxwell (1996). A Sexual Odyssey: From Forbidden Fruit to Cybersex. New York: Plenum. pp. 38–40. ISBN 030645405X.
- ↑ William Gervase Clarence-Smith (2000). Cocoa and Chocolate, 1765-1914. London: Routledge. pp. 10, 31. ISBN 0415215765.
- ↑ 28.0 28.1 US 20050089584, "Methods and compositions for oral delivery of Areca and mate' or theobromine"
- ↑ Usmani O (2005). "Theobromine inhibits sensory nerve activation and cough". FASEB J. 19 (2): 231–3. PMID 15548587. Unknown parameter
|coauthors=ignored (help) - ↑ 30.0 30.1 Irwin J. Polk (1997). All about Asthma: Stop Suffering and Start Living. New York: Insight Books. p. 100. ISBN 0306455692.
- ↑ Slattery M, West D (1993). "Smoking, alcohol, coffee, tea, caffeine, and theobromine: risk of prostate cancer in Utah (United States)". Cancer Causes Control. 4 (6): 559–63. PMID 8280834.
- ↑ "Theobromine" (in French). BIAM.
- ↑ Template:Harvard reference
- ↑ International Agency for Research on Cancer (November 17, 1991). "Volume 51: Coffee, Tea, Mate, Methylxanthines and Methylglyoxal - Theobromine" (PDF). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. WHO.
Further Reading
- Bender, David A. (1995). A Dictionary of Food and Nutrition. Oxford: Oxford University Press. ISBN 0198609612. Unknown parameter
|coauthors=ignored (help)
External Links
- Pages with script errors
- CS1 errors: ISBN
- Pages with citations using unsupported parameters
- CS1 maint: Multiple names: authors list
- CS1 maint: Unrecognized language
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles with erroneous molar mass calculations
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Xanthines
- Diuretics
- Cardiovascular Drugs
- Drug
- Vasodilators