Sturge-Weber syndrome
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Jesus Rosario Hernandez, M.D. [2] Rina Ghorpade, M.B.B.S[3]
Synonyms and keywords: Encephalotrigeminal angiomatosis; dimitri disease; encephalofacial angiomatosis; encephalotrigeminal angiomatosis; leptomeningeal angiomatosis; Sturge-Kalischer-Weber syndrome; Sturge-Weber-Krabbe syndrome; Sturge-Weber phakomatosis; SWS
Overview
Sturge-Weber syndrome(SWS), sometimes referred to as encephalotrigeminal angiomatosis, is an extremely rare congenital neurological and skin disorder. It is one of the phakomatoses, and is often associated with port-wine stains of the face, which is a capillary malformation, glaucoma, seizures, mental retardation, and ipsilateral leptomeningeal angioma. It is caused by an arteriovenous malformation that occurs in the cerebrum of the brain on the same side as the physical signs described above. Normally, only one side of the head is affected. It is an embryonal developmental anomaly resulting from errors in mesodermal and ectodermal development. Unlike other neurocutaneous disorders (phakomatoses), Sturge-Weber does not have a hereditary tendency but occurs sporadically. The diagnosis of SWS is established with neuroimaging techniques such as MRI with contrast, which shows the presence of leptomeningeal malformations in the brain. Treatment of this is symptomatic such as seizures are treated with anti-epileptic medications, ocular symptoms are managed with surgical modalities, and skin manifestations are treated with the laser. Prognosis of SWS is dependent on the age of onset of seizures, the extent of the brain, and dermatological involvement.
Historical Perspective
The term Sturge-Weber syndrome was coined by Professor Hilding Bergstrand in 1935 to acknowledge the work of two physicians, William Allen Sturge, and Parkes Weber. In 1879 William Allen Sturge noted a case of right sided facial lesion, ocular abnormalities, and neurological involvement in a six and half year old female. He later named this facial lesion as a port wine stain. In 1922, Parkes Weber, described the radiogical features of a brain lesion in a twenty-two year old woman with left sided vascular lesion on of her trunk, with right sided congenital spastic hemiplegia.[1] [2]
Classification
Encephalotrigeminal angiomatosis may be classified according to facial and leptomeningeal involvement into three subtypes by using Roach Scale. [3]
Type I: Classic Sturge-Weber syndrome that involves both facial, and leptomeningeal involvement, may have glaucoma.
Type II: Only facial angiomatosis with no CNS involvement, may have glaucoma.
Type III: Only CNS involvement, and no glaucoma.
Pathophysiology
The cause of SWS is somatic mosaic mutations in the GNAQ gene. The GNAQ gene is located on the long arm of chromosome number 9, at the position 21.2, and comprise of 7 exons. The function of this gene is to produce a protein called guanine nucleotide-binding protein G-alpha-q, which functions to regulate intracellular signaling pathways to help regulate the structure and functions of blood vessels. The somatic mutation in this gene (mutation that is occurring after conception and which is not inherited) replaces the amino acid arginine with the amino acid glutamine at position 183 in the G-alfa-q protein. This mutation specifically affects the cell responsible for angiogenesis in brain, skin and eyes. Other somatic mutation in GNAQ gene is responsible for the development of uveal melanoma, however, patients with SWS are not at high risk for the development of uveal melanoma.
On gross pathological examination, the leptomeninges looks thickened and the leptomeningeal vessels show dilatations and tortuosities. These vessels in leptomeninges show overexpression of Fibronectin and Vascular Endothelial Growth Factors (VEGF).[4]
On microscopic histopathological analysis of brain tissues show, intracranial calcification, gliosis, hypoplastic blood vessels, and loss of neurons. It is hypothesized that the hypoxic injury to the endothelial cells can lead to the breakdown of blood-brain barrier and can cause intracranial calcification. [5]
Causes
Clinical Features
Neurological manifestations
SWS is characterized by the formation of leptomeningeal capillary-venous malformation or angioma, which can cause various neurological symptoms such as seizures, stroke, intellectual disabilities, and behavioral issues. Intracranial angiomas occur on the same facial port-wine stain, it usually involves occipital and parietal lobes.[2]
- Seizures
Seizures is the most common symptom of SWS seen in 75-90% of patients , and it is often the first presentation. The age of onset of seizures is usually in early childhood, but it can occur at any age. The risk of seizures is high with a bilateral port-wine stain that unilateral port-wine stain.[6] At first, seizures are focal, but later in the course can turn into generalized tonic-clonic seizures. Rarely, a small subgroup of patients can present with infantile spasm and atonic seizures.
- Stroke like episodes
Possibly chronic hypoxia-induced injury in leptomeningeal blood vessels leads to stroke-like events. They occur in 25-60% of patients with SWS. These stroke-like episodes occur concurrently with the seizures and can lead to hemiparesis. Hemiparesis usually occur contralateral to the brain lesions. Children are more prone to develop these stroke-like episodes due to repeated falls and minor head injuries.[7].
- Cognitive impairment
Cognitive impairment in the form of delayed neuropsychological development occur in 50-60% patients with SWS, and it may range from mild attention deficit to severe deterioration in the mental capacity. Early-onset of seizures is associated with the high risk of developing severe forms of cognitive impairment.[6]. Other behavioral problems associated with SWS are autism spectrum disorder, social communication difficulties. [8]. Adults with normal cognitive function usually have depression, while aggression and self-destructive behavior occur in intellectually impaired adults.[5]
- Migraine like headaches
Headaches are very common symptoms of SWS Kossoff et al. reported the headaches can be as debilitating as seizures, the median age of onset of headache is 8. They also reported that the patients with a family history of headaches have an earlier age at onset of headache. [9]
- Neuroendocrine problems
A retrospective study conducted by Miller et al. concluded that the prevalence of the growth hormone deficiency is 18 fold higher than that of the general population.[10] The etiology of growth hormone deficiency in SWS is not well understood, interestingly, there is radiological undetectable structural damage to the pituitary gland. There is a higher incidence of central hypothyroidism in patients with SWS, due to an aberration in hypothalamic-pituitary axis resulting from neuronal abnormalities. [11] Therefore, patients with SWS should undergo screening tests such as thyroid function tests for central hypothyroidism and insulin-like growth factor-1 levels (IGF-1) to detect growth hormone deficiency.
Opthalmological issues
- Glaucoma
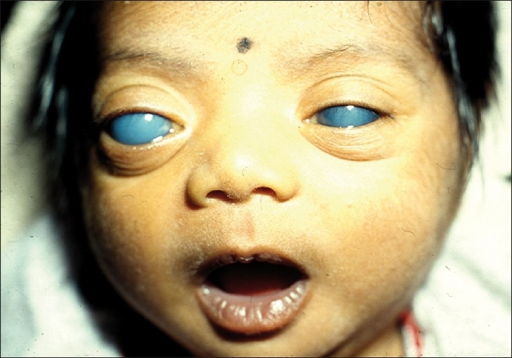
It is the most common ocular manifestation of SWS, and glaucoma occurs in nearly 70% of patients with SWS.[12] Usually glaucoma occurs ispilateral to the port-wine stain. The mechanism of pathogenesis of glaucoma are abnormality of an angle of anterior chamber which increases the resistance to the flow of aqueous humor, and higher venous pressure in episcleral veins due to formation of arteriovenous malformations especially in patients with episcleral hemangioma.[13]
Nearly 50% of patients with SWS present with buphthalmos (enlargement of an eye globe in newborns), and the risk of afflicted glaucoma is the highest in the first decade of life.
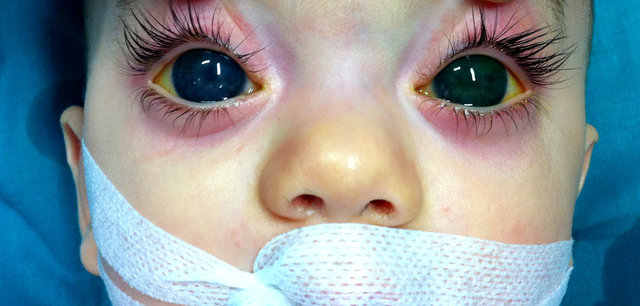
- Other Ocular Manifestations
Choroidal hemangioma present in nearly 50% of patients with SWS. Other ophthalmological problems in patients with SWS are retinal detachment, heterochromia iridium, and strabismus.
Port Wine Stain (PWS)
Port-wine stains are capillary malformations and are the most common skin manifestation of SWS. They are present at birth, usually occur on one side of the face, although these lesions can occur bilaterally. At birth, these stains are pink in color, however, the appearance of these stains changes with time, they grow dark red with increasing age. These lesions appear nodular and thick due to the dilatation of superficial vessels. PWS follows the distribution of three sensory divisions of trigeminal nerve, V1 the frontal branch, V2 the maxillary branch, and V3 the mandibular branch. In SWS the port-wine stains usually occur on the forehead, upper eyelid.[14][3]
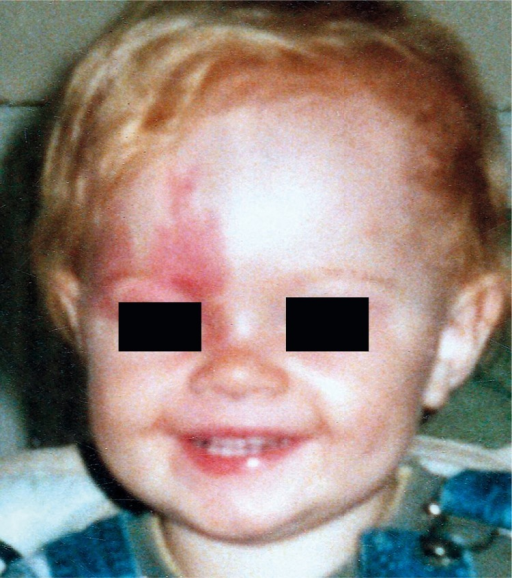
Port-wine stains are often misdiagnosed as a salmon patch or Nevus simplex or infantile hemangioma.
The following table distinguishes the common differential diagnosis of the port-wine stains.
| Features | Port-wine stain | Salmon patch | Infantile hemangioma |
|---|---|---|---|
| Other names | Nevus flammeus |
|
Hemangioma |
| Pathology | Capillary malformation | Capillary malformation | Vasular tumors |
| Color |
|
|
|
| Distribution |
|
|
Can occur anywhere in the skin, mucous
membrane, or internal organs. |
| Age at presentation | Present at birth | Present at birth | Not present at birth, but usually occur within a few months of life. |
| Progression |
|
|
Proliferation phase followed by involution |
| Association |
|
|
PHACE syndrome
H: Hemangiomas A: Arterial abnormalities C: Cardiac anomalies, coarctation of the aorta E: Endocrine and eye abnoramlities] |
Differentiating SWS from other disorders
The following disorders should be included in the differential diagnosis of SWS.
1) Klippel-Trenaunay-Weber syndrome is also a sporadic congenital disorder, this syndrome is characterized by Port-wine stain mostly on lower extremities, and hemihypertrophy of soft tissues. This syndrome rarely co-occurs with SWS.[15]
2) Beckwith-Wiedemann syndrome this syndrome is characterized by facial Port-wine stain, macroglossia, and hemihypertrophy.
Epidemiology and Demographics
The incidence of SWS according to the National Organization for Rare Disorders is approximately 1 in 20,000-50,000 live births. There is no documented sex or racial predilection found in the literature.
Risk Factors
Currently, SWS may be suspected in infants born with facial Port-wine stain particularly of the upper eyelid. Usually, 10-50% of newborns born with facial PWS have brain involvement. The risk of brain involvement is higher when facial Port-wine stain are bilateral.
Natural history, complication, and prognosis
The natural history of SWS is unpredictable, some patients have unmanagable seizures, while others do well.
Complications
Common complications of SWS include epilepsy, untreated glaucoma can cause vision loss, developmental delay, growth hormone deficiency , and hypothyroidism.
Prognosis
The prognosis of SWS depends on the following factors
1) Age at the onset of seizures: Earlier onset of seizures indicates a poorer prognosis and higher risk of neurological problems.
2) The extent of involvement of skin: Extensive involvement of skin indicates the poor prognosis.
3) The extent of involvement of the brain: Bilateral brain involvement indicates a high risk of development of learning disorders and intellectual disability.[16]
Diagnosis
SWS should be suspected in infants with Port-wine stain on the forehead and upper eyelid. To confirm the diagnosis of SWS the ophthalmological examination and the MRI with contrast should be performed.
Physical Examination
Physical examination may be remarkable for:
Laboratory Findings
There are no specific laboratory findings associated with SWS.
Imaging Findings
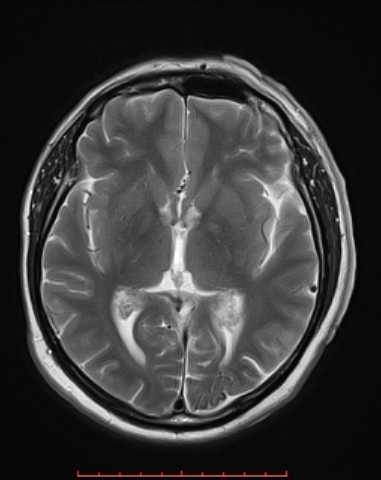
MRI with gadolinium contrast is the imaging modality of choice for SWS. , which can demonstrate the presence of leptomeningeal capillary malformations. It can also show the extent of brain involvement. The axial MRI below shows the volume loss in the left parietal and occipital lobes.
CT scan of the brain may be indicated in Emergency room to evaluate children presenting with seizures. It may demonstrate calcifications, loss of volume, enlarged ventricles, and tam track sign. The image below shows subcortical calcification seen in the region of left parietal and occipital lobes accompanied by parenchymal volume loss.
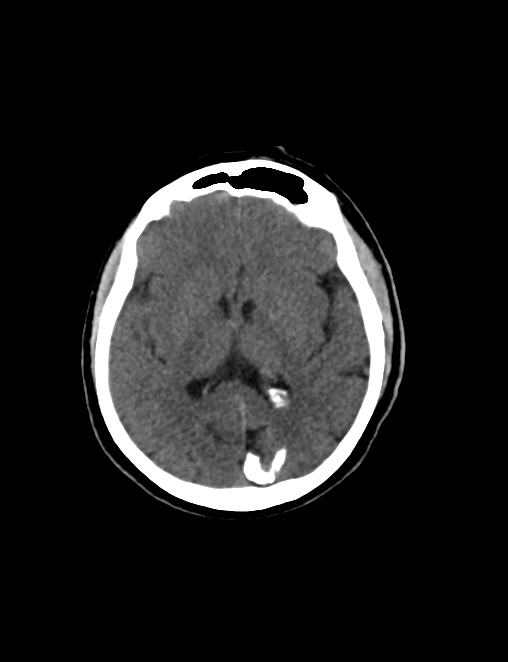
Treatment
There is no specific treatment available for SWS.
Seizures
Antiepileptics are the mainstay of the management of seizures associated with SWS. The most commonly used antiepileptics are phenobarbital, carbamazepine, oxcarbazepine, and levetiracetam. Carbamazepine and oxcarbazpine associated with the better seizure control than levetiracetam. [17] In untreated cases of intractable seizures surgery is indicated, through hemispherectomy, lobectomy or transection of corpus callosum.
Port-wine stain
For the facial Port-wine stain the treatment with pulsed dye laser is the treatment of choice.[18].
Glaucoma
The early-onset glaucoma can be treated with surgical methods like trabeculectomy or geniotomy. For the late-onset glaucoma the topical medications to lower the intraocular pressure are used. In newborns with a high risk of development of glaucoma should have an ophthalmological check-up every 3 months for the first year of life, and then annually throughout the life of an individual.
Patients with cognitive deficits may benefit from behavioral psychology.
Prevention
There are no primary preventive measures available for SWS.
The patients diagnosed with SWS should be started on the antiepileptic before the first seizure especially in those with extensive leptomeningeal involvement, the prophylactic use of antiepiletics may decrease the risk of cognitive impairment.[17]. Low-dose aspirin 3-5 mg/kg/day can be used to in all children diagnosed with SWS to lower the risk of hypoxic-ischemic neuronal injury, as aspirin has antithrombotic effects.[19]
References
- ↑ Weber FP (1922). "RIGHT-SIDED HEMI-HYPOTROPHY RESULTING FROM RIGHT-SIDED CONGENITAL SPASTIC HEMIPLEGIA, WITH A MORBID CONDITION OF THE LEFT SIDE OF THE BRAIN, REVEALED BY RADIOGRAMS". J Neurol Psychopathol. 3 (10): 134–9. doi:10.1136/jnnp.s1-3.10.134. PMC 1068054. PMID 21611493.
- ↑ 2.0 2.1 Sudarsanam A, Ardern-Holmes SL (2014). "Sturge-Weber syndrome: from the past to the present". Eur J Paediatr Neurol. 18 (3): 257–66. doi:10.1016/j.ejpn.2013.10.003. PMID 24275166.
- ↑ 3.0 3.1 Singh AK, Keenaghan M. PMID 29083797. Missing or empty
|title=(help) - ↑ Comati A, Beck H, Halliday W, Snipes GJ, Plate KH, Acker T (2007). "Upregulation of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha in leptomeningeal vascular malformations of Sturge-Weber syndrome". J Neuropathol Exp Neurol. 66 (1): 86–97. doi:10.1097/nen.0b013e31802d9011. PMID 17204940.
- ↑ 5.0 5.1 Comi AM (2011). "Presentation, diagnosis, pathophysiology, and treatment of the neurological features of Sturge-Weber syndrome". Neurologist. 17 (4): 179–84. doi:10.1097/NRL.0b013e318220c5b6. PMC 4487915. PMID 21712663.
- ↑ 6.0 6.1 Sujansky E, Conradi S (1995). "Sturge-Weber syndrome: age of onset of seizures and glaucoma and the prognosis for affected children". J Child Neurol. 10 (1): 49–58. doi:10.1177/088307389501000113. PMID 7769179.
- ↑ Zolkipli Z, Aylett S, Rankin PM, Neville BG (2007). "Transient exacerbation of hemiplegia following minor head trauma in Sturge-Weber syndrome". Dev Med Child Neurol. 49 (9): 697–9. doi:10.1111/j.1469-8749.2007.00697.x. PMID 17718827.
- ↑ Gittins S, Steel D, Brunklaus A, Newsom-Davis I, Hawkins C, Aylett SE (2018). "Autism spectrum disorder, social communication difficulties, and developmental comorbidities in Sturge-Weber syndrome". Epilepsy Behav. 88: 1–4. doi:10.1016/j.yebeh.2018.08.006. PMID 30195931.
- ↑ Kossoff EH, Hatfield LA, Ball KL, Comi AM (2005). "Comorbidity of epilepsy and headache in patients with Sturge-Weber syndrome". J Child Neurol. 20 (8): 678–82. doi:10.1177/08830738050200080901. PMID 16225815.
- ↑ Miller RS, Ball KL, Comi AM, Germain-Lee EL (2006). "Growth hormone deficiency in Sturge-Weber syndrome". Arch Dis Child. 91 (4): 340–1. doi:10.1136/adc.2005.082578. PMC 2065976. PMID 16551788.
- ↑ Comi AM, Bellamkonda S, Ferenc LM, Cohen BA, Germain-Lee EL (2008). "Central hypothyroidism and Sturge-Weber syndrome". Pediatr Neurol. 39 (1): 58–62. doi:10.1016/j.pediatrneurol.2008.03.018. PMID 18555176.
- ↑ Sullivan TJ, Clarke MP, Morin JD (1992). "The ocular manifestations of the Sturge-Weber syndrome". J Pediatr Ophthalmol Strabismus. 29 (6): 349–56. PMID 1287171.
- ↑ Abdolrahimzadeh, Solmaz; Scavella, Vittorio; Felli, Lorenzo; Cruciani, Filippo; Contestabile, Maria Teresa; Recupero, Santi Maria (2015). "Ophthalmic Alterations in the Sturge-Weber Syndrome, Klippel-Trenaunay Syndrome, and the Phakomatosis Pigmentovascularis: An Independent Group of Conditions?". BioMed Research International. 2015: 1–11. doi:10.1155/2015/786519. ISSN 2314-6133.
- ↑ Kolb L, Krishnamurthy K. PMID 29083716. Missing or empty
|title=(help) - ↑ Sakaguchi Y, Takenouchi T, Uehara T, Kishi K, Takahashi T, Kosaki K (2017). dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28782176 "Co-occurrence of Sturge-Weber syndrome and Klippel-Trenaunay-Weber syndrome phenotype: Consideration of the historical aspect" Check
|url=value (help). Am J Med Genet A. 173 (10): 2831–2833. doi:10.1002/ajmg.a.38363. PMID 28782176. - ↑ Bloom PJ (1977). "Determination of monaural sensitivity changes due to the pinna by use of minimum-audible-field measurements in the lateral vertical plane". J Acoust Soc Am. 61 (3): 820–8. doi:10.1121/1.381346. PMID 0853154.
- ↑ 17.0 17.1 Kaplan EH, Kossoff EH, Bachur CD, Gholston M, Hahn J, Widlus M; et al. (2016). "Anticonvulsant Efficacy in Sturge-Weber Syndrome". Pediatr Neurol. 58: 31–6. doi:10.1016/j.pediatrneurol.2015.10.015. PMC 4943018. PMID 26997037.
- ↑ Alster TS, Railan D (2006). "Laser treatment of vascular birthmarks". J Craniofac Surg. 17 (4): 720–3. doi:10.1097/00001665-200607000-00023. PMID 16877924.
- ↑ Lance EI, Sreenivasan AK, Zabel TA, Kossoff EH, Comi AM (2013). "Aspirin use in Sturge-Weber syndrome: side effects and clinical outcomes". J Child Neurol. 28 (2): 213–8. doi:10.1177/0883073812463607. PMC 4373084. PMID 23112247.
Pictures
Template:Phakomatoses and other congenital malformations not elsewhere classified