Insulin-like growth factor-I
| Insulin-like growth factor 1 (somatomedin C) | |||
|---|---|---|---|
 PDB rendering based on 1bqt. | |||
| Identifiers | |||
| Symbols | IGF1 ; IGFI | ||
| External IDs | Template:OMIM5 Template:MGI HomoloGene: 515 | ||
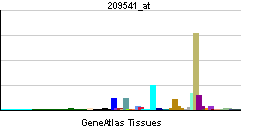
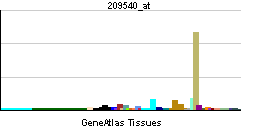
| RNA expression pattern | |||
 | |||
 | |||
 | |||
| More reference expression data | |||
| Orthologs | |||
| Template:GNF Ortholog box | |||
| Species | Human | Mouse | |
| Entrez | n/a | n/a | |
| Ensembl | n/a | n/a | |
| UniProt | n/a | n/a | |
| RefSeq (mRNA) | n/a | n/a | |
| RefSeq (protein) | n/a | n/a | |
| Location (UCSC) | n/a | n/a | |
| PubMed search | n/a | n/a | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Insulin-like growth factor 1 (IGF-1) that was once called somatomedin C, is a polypeptide protein hormone similar in molecular structure to insulin. It plays an important role in childhood growth and continues to have anabolic effects in adults.
Production and circulation
IGF-1 consists of 79 amino acids in a single chain with three intramolecular disulfide bridges. IGF-1 has a molecular weight of 7649 daltons. IGF-1 is produced primarily by the liver as an endocrine hormone as well as target tissues in a paracrine/autocrine fashion. Production is stimulated by growth hormone and can be retarded by undernutrition, growth hormone insensitivity, lack of growth hormone receptors, or failures of the downstream signalling pathway post GH receptor including SHP2 and STAT5b. Approximately 98% of IGF-1 is always bound to one of 6 binding proteins (IGF-BP). IGFBP-3, the most abundant protein, accounts for 80% of all IGF binding. IGF-1 binds to IGFBP-3 in a 1:1 molar ratio.
Action
Its primary action is mediated by binding to specific IGF receptors present on many cell types in many tissues. The signal is transduced by intracellular events. IGF-1 is one of the most potent natural activators of the AKT signaling pathway, a stimulator of cell growth and multiplication and a potent inhibitor of programmed cell death.
Almost every cell in the human body is affected by IGF-1, especially cells in muscle, cartilage, bone, liver, kidney, nerves, skin, and lungs. In addition to the insulin-like effects, IGF-1 can also regulate cell growth and development, especially in nerve cells, as well as cellular DNA synthesis.
IGF-1 is closely related to a second protein called "IGF-2". IGF-2 also binds the IGF-1 Receptor. However, IGF-2 alone binds a receptor called the "IGF II Receptor" (also called the Mannose-6 phosphate receptor). The insulin growth factor-II receptor (IGF2R) lacks signal transduction capacity, and its main role is to act as a sink for IGF-2 and make less IGF-2 available for binding with IGF-1R. As the name "insulin-like growth factor 1" implies, IGF-1 is structurally related to insulin, and is even capable of binding the insulin receptor, albeit at lower affinity than insulin.
Receptors
IGF-1 binds to at least two cell surface receptors: the IGF-1 receptor (IGFR), and the insulin receptor. The IGF-1 receptor seems to be the "physiologic" receptor - it binds IGF-1 at significantly higher affinity than IGF-1 is bound to the insulin receptor. Like the insulin receptor, the IGF-1 receptor is a receptor tyrosine kinase - meaning it signals by causing the addition of a phosphate molecule on particular tyrosines. IGF-1 activates the insulin receptor at approximately 0.1x the potency of insulin. Part of this signaling may be via IGF1R/Insulin Receptor heterodimers (the reason for the confusion is that binding studies show that IGF1 binds the insulin receptor 100-fold less well than insulin, yet that does not correlate with the actual potency of IGF1 in vivo at inducing phosphorylation of the insulin receptor, and hypoglycemia)..
IGF-1 is produced throughout life. The highest rates of IGF-1 production occur during the pubertal growth spurt. The lowest levels occur in infancy and old age.
Use as a diagnostic test
IGF-1 levels can be measured in the blood in 10-1000 ng/ml amounts. As levels do not fluctuate greatly throughout the day for an individual person, IGF-1 is used by physicians as a screening test for growth hormone deficiency and excess.
Interpretation of IGF-1 levels is complicated by the wide normal ranges, and variations by age, sex, and pubertal stage. Clinically significant conditions and changes may be masked by the wide normal ranges. Sequential management over time is often useful for the management of several types of pituitary disease, undernutrition, and growth problems.
Diseases of deficiency and resistance
Rare diseases characterized by inability to make or respond to IGF-1 produce a distinctive type of growth failure. One such disorder, termed Laron dwarfism does not respond at all to growth hormone treatment due to a lack of GH receptors. The FDA has grouped these diseases into a disorder called severe primary IGF deficiency. Patients with severe primary IGFD typically present with normal to high GH levels, height below -3 standard deviations (SD), and IGF-1 levels below -3SD. Severe primary IGFD includes patients with mutations in the GH receptor, post-receptor mutations or IGF mutations, as previously described. As a result, these patients cannot be expected to respond to GH treatment.
The IGF signaling pathway appears to play a crucial role in cancer. Several studies have shown that increased levels of IGF lead to an increased risk of cancer. Studies done on lung cancer cells show that drugs inhibiting such signaling can be of potential interest in cancer therapy.[1]
Factors influencing the levels of IGF-1 in the circulation
Factors that are known to cause variation in the levels of growth hormone (GH) and IGF-1 in the circulation include an individual's genetic make-up, the time of day, his or her age, gender, exercise status, stress levels, nutrition level and body mass index (BMI), disease state, race, estrogen status and xenobiotic intake.[2] The later inclusion of xenobiotic intake as a factor influencing GH-IGF status highlights the fact that the GH-IGF axis is a potential target for certain endocrine disrupting chemicals - see also endocrine disruptor.
IGF-1 as a therapeutic agent
IGF-1 has been manufactured recombinantly on a large scale using both yeast and E. coli. Several companies have evaluated IGF-1 in clinical trials for a variety of indications, including growth failure, type 1 diabetes, type 2 diabetes, amyotrophic lateral sclerosis (ALS aka "Lou Gehrig's Disease"), severe burn injury and myotonic muscular dystrophy (MMD). Results of clinical trials evaluating the efficacy of IGF-1 in type 1 diabetes and type 2 diabetes showed great promise in reducing hemoglobin A1C levels, as well as daily insulin consumption. However, the sponsor, Genentech, discontinued the program due to an exacerbation of diabetic retinopathy in patients coupled with a shift in corporate focus towards oncology. Cephalon and Chiron conducted two pivotal clinical studies of IGF-1 for ALS, and although one study demonstrated efficacy, the second was equivocal, and the product has never been approved by the FDA.
However, in the last few years, two additional companies Tercica and Insmed compiled enough clinical trial data to seek FDA approval in the United States. In August 2005, the FDA approved Tercica's IGF-1 drug, Increlex, as replacement therapy for severe primary IGF-1 deficiency based on clinical trial data from 71 patients. In December 2005, the FDA also approved Iplex, Insmed's IGF-1/IGFBP-3 complex. The Insmed drug is injected once a day versus the twice-a-day version that Tercica sells.
By delivering Iplex in a complex, patients can get the same efficacy with regard to growth rates but experience fewer side effects with less severe hypoglycemia. This would seem to make sense, since in the human body 97-99% of IGF-1 is bound to one of six IGF binding proteins. IGFBP-3 is the most abundant of these binding proteins, accounting for approximately 80% of IGF-1 binding.
Insmed was found to infringe on patents licensed by Tercica, which then sought to get a U.S. district court judge to ban sales of Iplex. [2] To settle patent infringement charges and resolve all litigation between the two companies, Insmed in March 2007 agreed to withdraw Iplex from the U.S. market, leaving Tercica's Increlex as the sole version of IGF-1 available in the United States. [3]
Terminology
IGF-1 has been known as "sulfation factor"[3] and its effects were termed "nonsuppressible insulin-like activity" (NSILA) in the 1970s. It was also known as "somatomedin C" in the 1980s.
Additional images
-
IGF-1
References
- ↑ Velcheti V, Govindan R (2006). "Insulin-like growth factor and lung cancer". Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 1 (7): 607–10. PMID 17409926.
- ↑ Scarth J (2006). "Modulation of the growth hormone-insulin-like growth factor (GH-IGF) axis by pharmaceutical, nutraceutical and environmental xenobiotics: an emerging role for xenobiotic-metabolizing enzymes and the transcription factors regulating their expression. A review". Xenobiotica. 36 (2–3): 119–218. PMID 16702112.
- ↑ Salmon W, Daughaday W (1957). "A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro". J Lab Clin Med. 49 (6): 825–36. PMID 13429201.
Further reading
- Butler AA, Yakar S, LeRoith D (2002). "Insulin-like growth factor-I: compartmentalization within the somatotropic axis?". News Physiol. Sci. 17: 82–5. PMID 11909998.
- Maccario M, Tassone F, Grottoli S; et al. (2002). "Neuroendocrine and metabolic determinants of the adaptation of GH/IGF-I axis to obesity". Ann. Endocrinol. (Paris). 63 (2 Pt 1): 140–4. PMID 11994678.
- Camacho-Hübner C, Woods KA, Clark AJ, Savage MO (2003). "Insulin-like growth factor (IGF)-I gene deletion". Reviews in endocrine & metabolic disorders. 3 (4): 357–61. PMID 12424437.
- Trojan LA, Kopinski P, Wei MX; et al. (2004). "IGF-I: from diagnostic to triple-helix gene therapy of solid tumors". Acta Biochim. Pol. 49 (4): 979–90. doi:024904979 Check
|doi=value (help). PMID 12545204. - Winn N, Paul A, Musaró A, Rosenthal N (2003). "Insulin-like growth factor isoforms in skeletal muscle aging, regeneration, and disease". Cold Spring Harb. Symp. Quant. Biol. 67: 507–18. PMID 12858577.
- Delafontaine P, Song YH, Li Y (2005). "Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels". Arterioscler. Thromb. Vasc. Biol. 24 (3): 435–44. doi:10.1161/01.ATV.0000105902.89459.09. PMID 14604834.
- Trejo JL, Carro E, Garcia-Galloway E, Torres-Aleman I (2004). "Role of insulin-like growth factor I signaling in neurodegenerative diseases". J. Mol. Med. 82 (3): 156–62. doi:10.1007/s00109-003-0499-7. PMID 14647921.
- Rabinovsky ED (2004). "The multifunctional role of IGF-1 in peripheral nerve regeneration". Neurol. Res. 26 (2): 204–10. doi:10.1179/016164104225013851. PMID 15072640.
- Rincon M, Muzumdar R, Atzmon G, Barzilai N (2005). "The paradox of the insulin/IGF-1 signaling pathway in longevity". Mech. Ageing Dev. 125 (6): 397–403. PMID 15272501.
- Conti E, Carrozza C, Capoluongo E; et al. (2005). "Insulin-like growth factor-1 as a vascular protective factor". Circulation. 110 (15): 2260–5. doi:10.1161/01.CIR.0000144309.87183.FB. PMID 15477425.
- Wood AW, Duan C, Bern HA (2005). "Insulin-like growth factor signaling in fish". Int. Rev. Cytol. 243: 215–85. doi:10.1016/S0074-7696(05)43004-1. PMID 15797461.
- Sandhu MS (2005). "Insulin-like growth factor-I and risk of type 2 diabetes and coronary heart disease: molecular epidemiology". Endocrine development. 9: 44–54. doi:10.1159/000085755. PMID 15879687.
- Ye P, D'Ercole AJ (2006). "Insulin-like growth factor actions during development of neural stem cells and progenitors in the central nervous system". J. Neurosci. Res. 83 (1): 1–6. doi:10.1002/jnr.20688. PMID 16294334.
- Gómez JM (2006). "The role of insulin-like growth factor I components in the regulation of vitamin D.". Current pharmaceutical biotechnology. 7 (2): 125–32. doi:10.2174/138920106776597621. PMID 16724947.
- Federico G, Street ME, Maghnie M; et al. (2006). "Assessment of serum IGF-I concentrations in the diagnosis of isolated childhood-onset GH deficiency: a proposal of the Italian Society for Pediatric Endocrinology and Diabetes (SIEDP/ISPED)". J. Endocrinol. Invest. 29 (8): 732–7. PMID 17033263.
- Zakula Z, Koricanac G, Putnikovic B; et al. (2007). "Regulation of the inducible nitric oxide synthase and sodium pump in type 1 diabetes". Med. Hypotheses. 69 (2): 302–6. doi:10.1016/j.mehy.2006.11.045. PMID 17289286.
- Trojan J, Cloix JF, Ardourel MY; et al. (2007). "Insulin-like growth factor type I biology and targeting in malignant gliomas". Neuroscience. 145 (3): 795–811. doi:10.1016/j.neuroscience.2007.01.021. PMID 17320297.
External links
- Insulin-Like+Growth+Factor+I at the US National Library of Medicine Medical Subject Headings (MeSH)
- IGF-1 for Parents
- IGF-1 & Bodybuilding
- IGF1 Deficiency for Parents a section of The MAGIC Foundation for Children's Growth
da:IGF-1 he:IGF-1 nl:IGF1 sr:Инсулину сличан фактор раста 1 uk:Інсуліноподібний фактор росту 1