Posaconazole clinical pharmacology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
Clinical Pharmacology
Mechanism of Action
Posaconazole is an azole antifungal agent.
Pharmacodynamics
Exposure Response Relationship: In clinical studies of neutropenic patients who were receiving cytotoxic chemotherapy for acute myelogenous leukemia (AML) or myelodysplastic syndromes (MDS) or hematopoietic stem cell transplant (HSCT) recipients with Graft versus Host Disease (GVHD), a wide range of plasma exposures to posaconazole was noted. A pharmacokinetic-pharmacodynamic analysis of patient data revealed an apparent association between average posaconazole concentrations (Cav) and prophylactic efficacy (Table 8). A lower Cav may be associated with an increased risk of treatment failure, defined as treatment discontinuation, use of empiric systemic antifungal therapy (SAF), or occurrence of breakthrough invasive fungal infections.
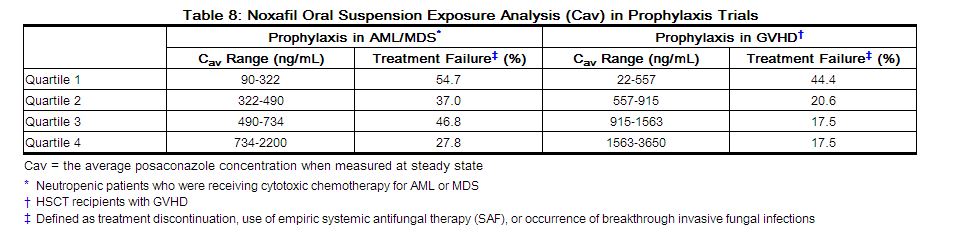 |
Pharmacokinetics
General Pharmacokinetic Characteristics
Noxafil delayed-release tablets exhibit dose proportional pharmacokinetics after single and multiple dosing up to 300 mg. The mean pharmacokinetic parameters of posaconazole at steady state following administration of Noxafil delayed-release tablets 300 mg twice daily (BID) on Day 1, then 300 mg once daily (QD) thereafter in healthy volunteers and in neutropenic patients who are receiving cytotoxic chemotherapy for AML or MDS or HSCT recipients with GVHD are shown in Table 9.
| 600px|thumb]] |
Dose-proportional increases in plasma exposure (AUC) to posaconazole oral suspension were observed following single oral doses from 50 mg to 800 mg and following multiple-dose administration from 50 mg BID to 400 mg BID in healthy volunteers. No further increases in exposure were observed when the dose of the oral suspension increased from 400 mg BID to 600 mg BID in febrile neutropenic patients or those with refractory invasive fungal infections.
The mean (%CV) [min-max] posaconazole oral suspension average steady-state plasma concentrations (Cav) and steady-state pharmacokinetic parameters in patients following administration of 200 mg TID and 400 mg BID of the oral suspension are provided in Table 10.
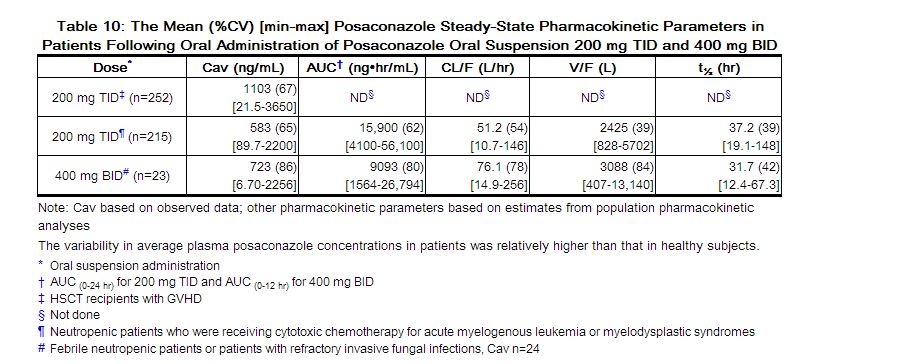 |
Absorption: When given orally in healthy volunteers, posaconazole delayed-release tablets are absorbed with a median Tmax of 4 to 5 hours. Steady-state plasma concentrations are attained by Day 6 at the 300 mg dose (QD after BID loading dose at Day 1). The absolute bioavailability of the oral delayed-release tablet is approximately 54%. The effect of food intake on the oral bioavailability of posaconazole following administration of posaconazole delayed-release tablets is not known. However, since the oral bioavailability of posaconazole is significantly increased when the oral suspension is administered with food or a nutritional supplement (see below), it is also recommended that posaconazole delayed-release tablets be taken with food.
Concomitant administration of posaconazole delayed-release tablets with drugs affecting gastric pH or gastric motility did not demonstrate any significant effects on posaconazole pharmacokinetic exposure (see Table 11).
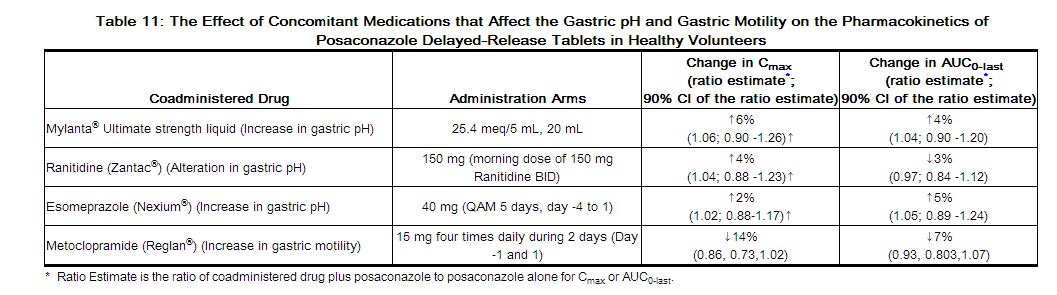 |
Posaconazole oral suspension is absorbed with a median Tmax of ~3 to 5 hours. Steady-state plasma concentrations are attained at 7 to 10 days following multiple-dose administration.
Following single-dose administration of 200 mg, the mean AUC and Cmax of posaconazole are approximately 3-times higher when the oral suspension is administered with a nonfat meal and approximately 4-times higher when administered with a high-fat meal (~50 gm fat) relative to the fasted state. Following single-dose administration of posaconazole oral suspension 400 mg, the mean AUC and Cmax of posaconazole are approximately 3-times higher when administered with a liquid nutritional supplement (14 gm fat) relative to the fasted state (see Table 12). In addition, the effects of varying gastric administration conditions on the Cmax and AUC of posaconazole oral suspension in healthy volunteers have been investigated and are shown in Table 13.
In order to assure attainment of adequate plasma concentrations, it is recommended to administer Noxafil oral suspension during or immediately following a full meal. In patients who cannot eat a full meal, Noxafil oral suspension should be taken with a liquid nutritional supplement or an acidic carbonated beverage (e.g., ginger ale).
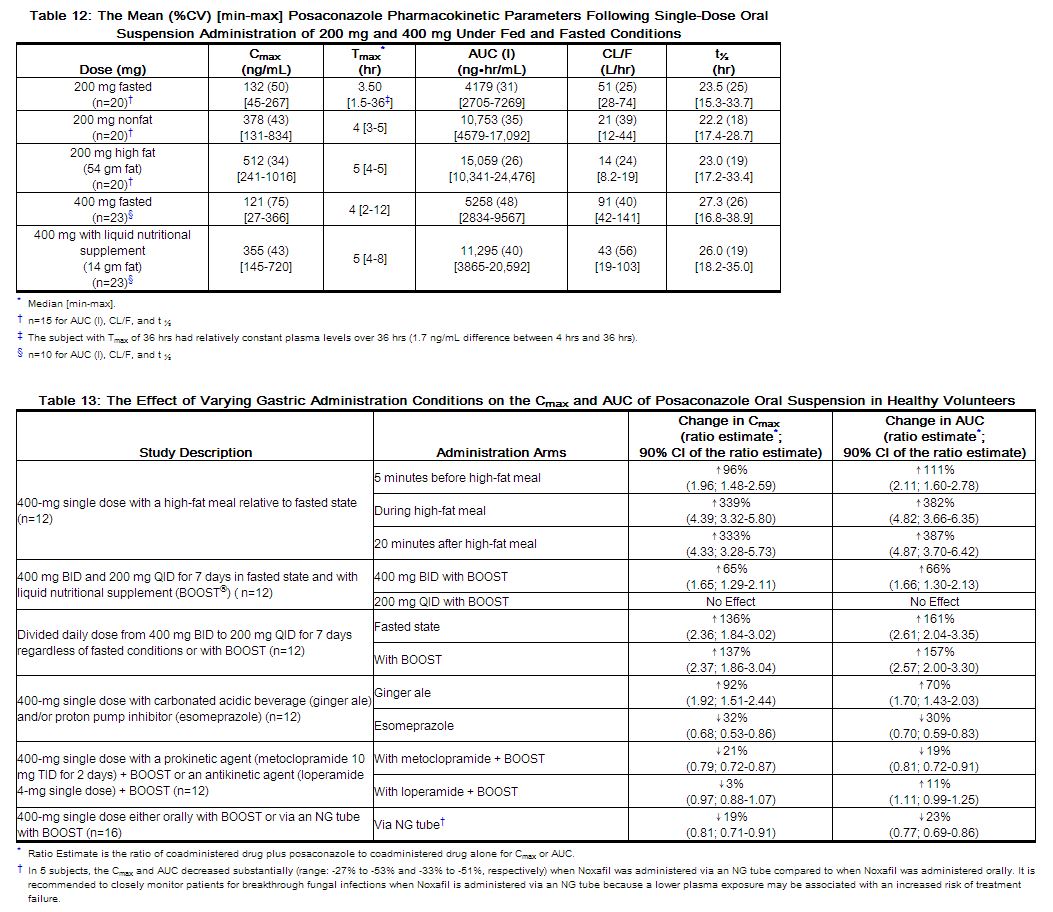 |
Concomitant administration of posaconazole oral suspension with drugs affecting gastric pH or gastric motility results in lower posaconazole exposure. (See Table 14.)
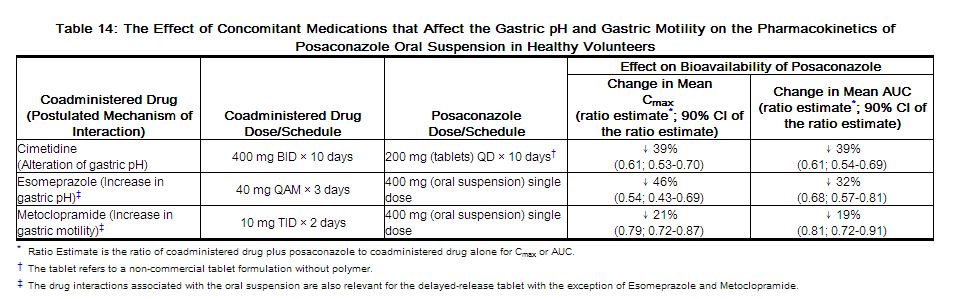 |
Distribution: Posaconazole has a mean (CV%) volume of distribution of 287 L (24%) in healthy volunteers. Posaconazole is highly bound to human plasma proteins (>98%), predominantly to albumin.
Metabolism: Posaconazole primarily circulates as the parent compound in plasma. Of the circulating metabolites, the majority are glucuronide conjugates formed via UDP glucuronidation (phase 2 enzymes). Posaconazole does not have any major circulating oxidative (CYP450 mediated) metabolites. The excreted metabolites in urine and feces account for ~17% of the administered radiolabeled dose.
Posaconazole is primarily metabolized via UDP glucuronidation (phase 2 enzymes) and is a substrate for p-glycoprotein (P-gp) efflux. Therefore, inhibitors or inducers of these clearance pathways may affect posaconazole plasma concentrations. A summary of drugs studied clinically, which affect posaconazole concentrations, is provided in Table 15.
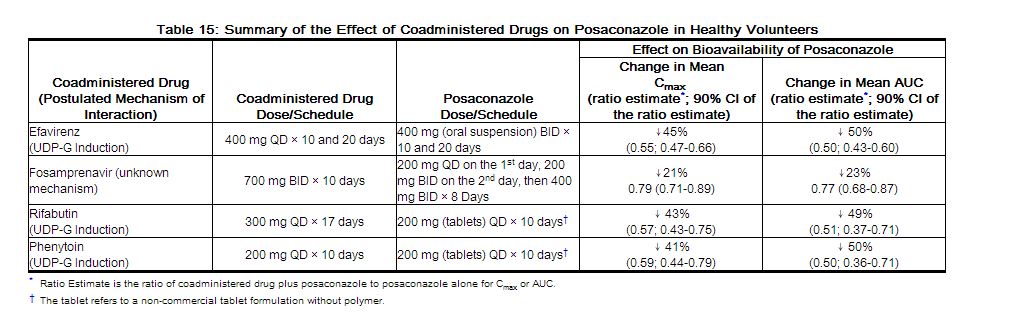 |
In vitro studies with human hepatic microsomes and clinical studies indicate that posaconazole is an inhibitor primarily of CYP3A4. A clinical study in healthy volunteers also indicates that posaconazole is a strong CYP3A4 inhibitor as evidenced by a >5-fold increase in midazolam AUC. Therefore, plasma concentrations of drugs predominantly metabolized by CYP3A4 may be increased by posaconazole. A summary of the drugs studied clinically, for which plasma concentrations were affected by posaconazole, is provided in Table 16 [see Contraindications (4) and Drug Interactions (7.1) including recommendations].
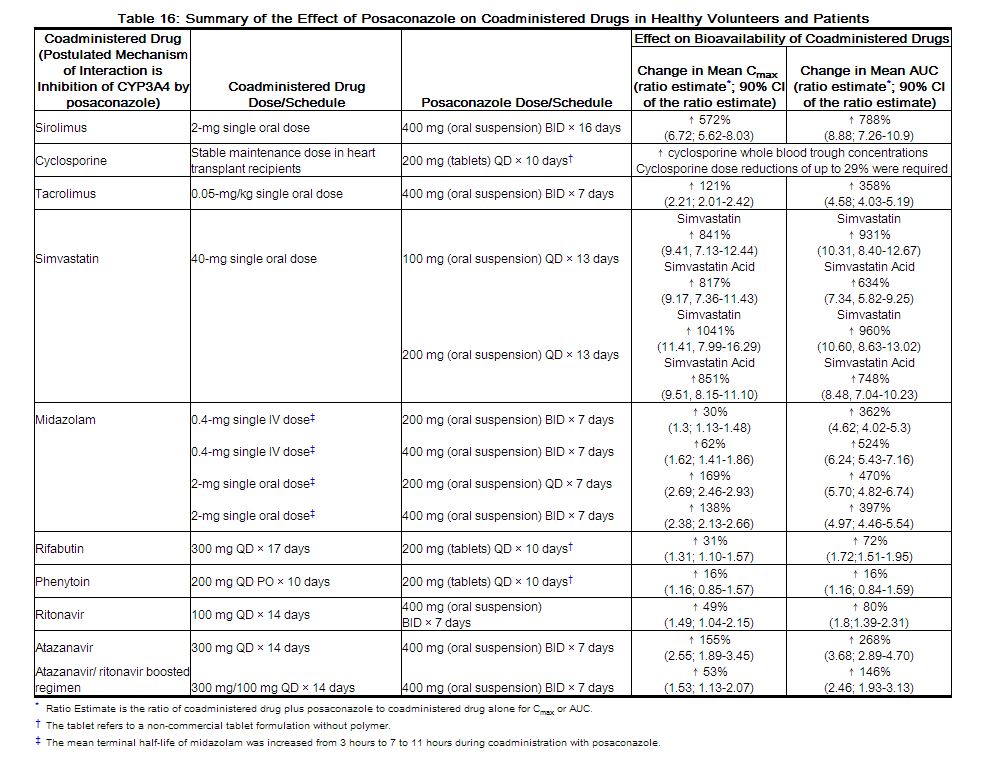 |
Additional clinical studies demonstrated that no clinically significant effects on zidovudine, lamivudine, indinavir, or caffeine were observed when administered with posaconazole 200 mg QD; therefore, no dose adjustments are required for these coadministered drugs when coadministered with posaconazole 200 mg QD.
Excretion: Posaconazole is predominantly eliminated in the feces (71% of the radiolabeled dose up to 120 hours) with the major component eliminated as parent drug (66% of the radiolabeled dose). Renal clearance is a minor elimination pathway, with 13% of the radiolabeled dose excreted in urine up to 120 hours (<0.2% of the radiolabeled dose is parent drug).
Posaconazole delayed-release tablet is eliminated with a mean half-life (t½) ranging between 26 to 31 hours.
Posaconazole oral suspension is eliminated with a mean half-life (t½) of 35 hours (range: 20-66 hours).[1]
References
Adapted from the FDA Package Insert.