Penicillin G sodium microbiology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mohamed Moubarak, M.D. [2]
Microbiology
Penicillin G is bactericidal against penicillin-susceptible microorganisms during the stage of active multiplication. It acts by inhibiting biosynthesis of cell-wall mucopeptide. It is not active against the penicillinase-producing bacteria, which include many strains of staphylococci. Penicillin G is highly active in vitro against staphylococci (except penicillinase-producing strains), streptococci (groups A, B, C, G, H, L and M), pneumococci and Neisseria meningitidis. Other organisms susceptible in vitro to penicillin G are Neisseria gonorrhoeae, Corynebacterium diphtheriae, Bacillus anthracis, clostridia, Actinomyces species, Spirillum minus, Streptobacillus monillformis, Listeria monocytogenes, and leptospira; Treponema pallidum is extremely susceptible.
Some species of gram-negative bacilli were previously considered susceptible to very high intravenous doses of penicillin G (up to 80 million units/day) including some strains of Escherichia coli, Proteus mirabilis, salmonella, shigella, Enterobacteraerogenes (formerly Aerobacteraerogenes) and Alcaligenesfaecalis. Penicillin G is no longer considered a drug of choice for infections caused by these organisms.[1]
Susceptibility Testing
- Diffusion techniques
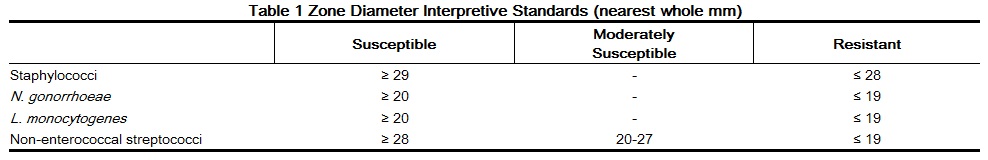
The use of antibiotic disk susceptibility test methods which measure zone diameter give an accurate estimation of antibiotic susceptibility. One such standard procedure1 which has been recommended for use with disks to test susceptibility of organisms to penicillin G uses the 10 Unit (U) penicillin disk. Interpretation involves the correlation of the diameters obtained in the disk test with the minimum inhibitory concentration (MIC) for penicillin G.
Reports from the laboratory giving results of the standard single-disk susceptibility test with a 10 U penicillin disk should be interpreted according to the following criteria:
A report of “susceptible” indicates that the pathogen is likely to be inhibited by generally achievable blood levels. A report of “moderately susceptible” suggests that the organism would be susceptible if high dosage is used or if the infection is confined to tissue and fluids (e.g., urine) in which high antibiotic levels are obtained. A report of “resistant” indicates that achievable concentrations are unlikely to be inhibitory and other therapy should be selected.
Standardized procedures require the use of laboratory control organisms. The 10 U penicillin G disk should give the following zone diameters:
- Organism Staphylococcus aureus ATCC 25923
- Zone Diameter (mm) 26-37
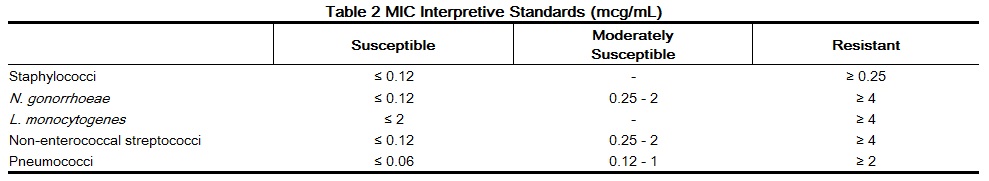
- Dilution techniques
When using a standardized dilution method2 (broth, agar, microdilution) or equivalent an organism may be considered susceptible if the minimum inhibitory concentration (MIC) values are interpreted according to the following table:
MIC test results should be interpreted according to the concentration of penicillin G that can be attained in blood (serum), tissue, and body fluids. As with standard diffusion techniques, dilution methods require the use of laboratory control organisms. Standard penicillin G powder should provide the following MIC values:
For anaerobic bacteria the MIC of penicillin G can be determined by agar or broth dilution (including microdilution) techniques.
References
- ↑ "PENICILLIN G SODIUM INJECTION, POWDER, FOR SOLUTION [SANDOZ INC]". Text " accessdate" ignored (help)
Adapted from the FDA Package Insert.