Paclitaxel
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2];Aparna Vuppala, M.B.B.S. [3]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Neutropenia
See full prescribing information for complete Boxed Warning.
* Do not administer paclitaxel therapy to patients who have baseline neutrophil counts of less than 1,500 cells/mm3. In order to monitor the occurrence of bone marrow suppression, primarily neutropenia, which may be severe and result in infection, it is recommended that frequent peripheral blood cell counts be performed on all patients receiving paclitaxel.
|
Overview
Paclitaxel is a mitotic inhibitor that is FDA approved for the treatment of metastatic breast cancer, non-small cell lung cancer and adenocarcinoma of the pancreas. There is a Black Box Warning for this drug as shown here. Common adverse reactions include alopecia, diarrhea, inflammatory disease of mucous membrane, nausea and vomiting, any grade of anemia, leukopenia, any grade of neutropenia, any grade of thrombocytopenia, any grade of hypersensitivity reaction, arthralgia, myalgia and peripheral neuropathy.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Metastatic Breast Cancer
- Paclitaxel is indicated for the treatment of breast cancer after failure of combination chemotherapy for metastatic disease or relapse within 6 months of adjuvant chemotherapy. Prior therapy should have included an anthracycline unless clinically contraindicated.
- Dosage: 260 mg/m2 administered intravenously over 30 minutes every 3 weeks
Non-Small Cell Lung Cancer
- Paclitaxel is indicated for the first-line treatment of locally advanced or metastatic non-small cell lung cancer, in combination with carboplatin, in patients who are not candidates for curative surgery or radiation therapy.
- Dosage: 100 mg/m2 administered as an intravenous infusion over 30 minutes on Days 1, 8, and 15 of each 21-day cycle.
- Administer carboplatin on Day 1 of each 21 day cycle immediately after paclitaxel
Adenocarcinoma of the Pancreas
- Paclitaxel is indicated for the first-line treatment of patients with metastatic adenocarcinoma of the pancreas, in combination with gemcitabine.
- Dosage: 125 mg/m2 administered as an intravenous infusion over 30-40 minutes on Days 1, 8 and 15 of each 28-day cycle.
- Administer gemcitabine immediately after paclitaxel on Days 1, 8 and 15 of each 28-day cycle
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Paclitaxel in adult patients.
Non–Guideline-Supported Use
- Angiosarcoma
- Breast cancer
- Cancer of unknown origin
- Carcinoma of bladder
- Carcinoma of esophagus
- In combination with carboplatin or cisplatin in carcinoma of fallopian tube
- Carcinoma of prostate
- Cervical cancer
- Gastric cancer
- Head and neck cancer
- Malignant lymphoma
- Malignant neoplasm of endometrium of corpus uteri
- Malignant tumor of nasopharynx
- In combination with carboplatin or cisplatin in malignant tumor of peritoneum
- Multiple myeloma of ovarian origin
- Non-small cell lung cancer
- Non-small cell lung cancer, First-line treatment in combination with bevacizumab and carboplatin for advanced/metastatic non-squamous cell disease
- Oligodendroglioma of brain
- Ovarian cancer
- Small cell carcinoma of lung
- Testicular cancer
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Paclitaxel FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Paclitaxel in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Paclitaxel in pediatric patients.
Contraindications
- Paclitaxel should not be used in patients who have baseline neutrophil counts of < 1,500 cells/mm3.
- Patients who experience a severe hypersensitivity reaction to paclitaxel should not be rechallenged with the drug.
Warnings
|
Neutropenia
See full prescribing information for complete Boxed Warning.
* Do not administer paclitaxel therapy to patients who have baseline neutrophil counts of less than 1,500 cells/mm3. In order to monitor the occurrence of bone marrow suppression, primarily neutropenia, which may be severe and result in infection, it is recommended that frequent peripheral blood cell counts be performed on all patients receiving paclitaxel.
|
Hematologic Effects
- Bone marrow suppression (primarily neutropenia) is dose-dependent and a dose-limiting toxicity of paclitaxel.
- In clinical studies, Grade 3-4 neutropenia occurred in 34% of patients with metastatic breast cancer (MBC), 47% of patients with non-small cell lung cancer (NSCLC), and 38% of patients with pancreatic cancer.
- Monitor for myelotoxicity by performing complete blood cell counts frequently, including prior to dosing on Day 1 (for MBC) and Days 1, 8, and 15 (for NSCLC and for pancreatic cancer).
- Do not administer paclitaxel to patients with baseline absolute neutrophil counts (ANC) of less than 1,500 cells/mm3. In the case of severe neutropenia (<500 cells/mm3 for seven days or more) during a course of paclitaxel therapy, reduce the dose of paclitaxel in subsequent courses in patients with either MBC or NSCLC.
- In patients with MBC, resume treatment with every-3-week cycles of paclitaxel after ANC recovers to a level >1,500 cells/mm3 and platelets recover to a level >100,000 cells/mm3.
- In patients with NSCLC, resume treatment if recommended at permanently reduced doses for both weekly paclitaxel and every-3-week carboplatin after ANC recovers to at least 1500 cells/mm3 and platelet count of at least 100,000 cells/mm3 on Day 1 or to an ANC of at least 500 cells/mm3 and platelet count of at least 50,000 cells/mm3 on Days 8 or 15 of the cycle.
- In patients with adenocarcinoma of the pancreas, withhold paclitaxel and gemcitabine if the ANC is less than 500 cells/mm3 or platelets are less than 50,000 cells/mm3 and delay initiation of the next cycle if the ANC is less than 1500 cells/mm3 or platelet count is less than 100,000 cells/mm3 on Day 1 of the cycle. Resume treatment with appropriate dose reduction if recommended.
Nervous System
- Sensory neuropathy is dose- and schedule-dependent.
- The occurrence of Grade 1 or 2 sensory neuropathy does not generally require dose modification.
- If ≥ Grade 3 sensory neuropathy develops, withhold paclitaxel treatment until resolution to Grade 1 or 2 for metastatic breast cancer or until resolution to ≤ Grade 1 for NSCLC and pancreatic cancer followed by a dose reduction for all subsequent courses of paclitaxel
Sepsis
- Sepsis occurred in 5% of patients with or without neutropenia who received paclitaxel in combination with gemcitabine. Biliary obstruction or presence of biliary stent were risk factors for severe or fatal sepsis.
- If a patient becomes febrile (regardless of ANC) initiate treatment with broad spectrum antibiotics.
- For febrile neutropenia, interrupt paclitaxel and gemcitabine until fever resolves and ANC ≥ 1500, then resume treatment at reduced dose levels.
Pneumonitis
- Pneumonitis, including some cases that were fatal, occurred in 4% of patients receiving paclitaxel in combination with gemcitabine.
- Monitor patients for signs and symptoms of pneumonitis and interrupt paclitaxel and gemcitabine during evaluation of suspected pneumonitis. After ruling out infectious etiology and upon making a diagnosis of pneumonitis, permanently discontinue treatment with paclitaxel and gemcitabine.
Hypersensitivity
- Severe and sometimes fatal hypersensitivity reactions, including anaphylactic reactions, have been reported. Patients who experience a severe hypersensitivity reaction to paclitaxel should not be re-challenged with this drug.
Albumin (Human)
- Paclitaxel contains albumin (human). Based on effective donor screening and product manufacturing processes, it carries a remote risk for transmission of viral diseases
- A theoretical risk for transmission of Creutzfeldt-Jakob Disease (CJD) also is considered extremely remote.
- No cases of transmission of viral diseases or CJD have ever been identified for albumin.
Adverse Reactions
Clinical Trials Experience
- The most common adverse reactions (≥ 20%) with single-agent use of paclitaxel in metastatic breast cancer are alopecia, neutropenia, sensory neuropathy, abnormal ECG, fatigue/asthenia, myalgia/arthralgia, AST elevation, alkaline phosphatase elevation, anemia, nausea, infections, and diarrhea.
- The most common adverse reactions (≥ 20%) of paclitaxel in combination with carboplatin for non-small cell lung cancer are anemia, neutropenia, thrombocytopenia, alopecia, peripheral neuropathy, nausea, and fatigue.
- The most common serious adverse reactions of paclitaxel in combination with carboplatin for non-small cell lung cancer are anemia (4%) and pneumonia (3%).
- The most common adverse reactions resulting in permanent discontinuation of paclitaxel are neutropenia (3%), thrombocytopenia (3%), and peripheral neuropathy (1%).
- The most common adverse reactions resulting in dose reduction of paclitaxel are neutropenia (24%), thrombocytopenia (13%), and anemia (6%).
- The most common adverse reactions leading to withholding or delay in paclitaxel dosing are neutropenia (41%), thrombocytopenia (30%), and anemia (16%).
- In a randomized open-label trial of paclitaxel in combination with gemcitabine for pancreatic adenocarcinoma, the most common (≥ 20%) selected (with a ≥ 5% higher incidence) adverse reactions of paclitaxel are neutropenia, fatigue, peripheral neuropathy, nausea, alopecia, peripheral edema, diarrhea, pyrexia, vomiting, decreased appetite, rash, and dehydration.
- The most common serious adverse reactions of paclitaxel (with a ≥ 1% higher incidence) are pyrexia (6%), dehydration (5%), pneumonia (4%) and vomiting (4%).
- The most common adverse reactions resulting in permanent discontinuation of paclitaxel are peripheral neuropathy (8%), fatigue (4%) and thrombocytopenia (2%).
- The most common adverse reactions resulting in dose reduction of paclitaxel are neutropenia (10%) and peripheral neuropathy (6%).
- The most common adverse reactions leading to withholding or delay in paclitaxel dosing are neutropenia (16%), thrombocytopenia (12%), fatigue (8%), peripheral neuropathy (15%), anemia (5%) and diarrhea (5%).
Postmarketing Experience
Hypersensitivity Reactions
- Severe and sometimes fatal hypersensitivity reactions have been reported with paclitaxel. The use of paclitxel in patients previously exhibiting hypersensitivity to paclitaxel injection or human albumin has not been studied.
Cardiovascular
- There have been reports of congestive heart failure, left ventricular dysfunction, and atrioventricular block with paclitaxel.
- Most of the individuals were previously exposed to cardiotoxic drugs ,such as anthracyclines, or had underlying cardiac history.
Respiratory
- There have been reports of pneumonitis, interstitial pneumonia and pulmonary embolism in patients receiving paclitaxel and reports of radiation pneumonitis in patients receiving concurrent radiotherapy.
- Reports of lung fibrosis have been received as part of the continuing surveillance of paclitaxel injection safety and may also be observed with paclitaxel.
Neurologic
- Cranial nerve palsies and vocal cord paresis have been reported, as well as autonomic neuropathy resulting in paralytic ileus.
Vision Disorders
- Reports in the literature of abnormal visual evoked potentials in patients treated with paclitaxel injection suggest persistent optic nerve damage.
- Reduced visual acuity due to cystoid macular edema (CME)
- After cessation of treatment, CME improves and visual acuity may return to baseline.
Hepatic
- Reports of hepatic necrosis and hepatic encephalopathy leading to death
Gastrointestinal
- Intestinal obstruction
- Intestinal perforation
- Pancreatitis
- Ischemic colitis
- Neutropenic enterocolitis (typhlitis)
Injection Site Reaction
- Severe events such as phlebitis, cellulitis, induration, necrosis, and fibrosis have been reported as part of the continuing surveillance of paclitaxel injection safety.
- In some cases the onset of the injection site reaction in paclitaxel injection patients either occurred during a prolonged infusion or was delayed by a week to ten days.
- Recurrence of skin reactions at a site of previous extravasation following administration of paclitaxel injection at a different site, i.e., “recall”, has been reported.
Other Clinical Events
- Skin reactions including generalized or maculopapular rash, erythema, and pruritus.
- Photosensitivity reactions
- Radiation recall phenomenon
- In some patients previously exposed to capecitabine, reports of palmar-plantar erythrodysesthesia
- Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported
- Conjunctivitis
- Cellulitis
- Increased lacrimation
Drug Interactions
- The metabolism of paclitaxel is catalyzed by CYP2C8 and CYP3A4.
- Caution should be exercised when administering paclitaxel concomitantly with medicines known to inhibit (e.g., ketoconazole and other imidazole antifungals, erythromycin, fluoxetine, gemfibrozil, cimetidine, ritonavir, saquinavir, indinavir, and nelfinavir) or induce (e.g., rifampicin, carbamazepine, phenytoin, efavirenz, and nevirapine) either CYP2C8 or CYP3A4.
Use in Specific Populations
Pregnancy
- There are no adequate and well-controlled studies in pregnant women using paclitaxel. Based on its mechanism of action and findings in animals, paclitaxel can cause fetal harm when administered to a pregnant woman. If this drug is used during pregnancy, or if the patient becomes pregnant while receiving this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant while receiving paclitaxel.
- Administration of paclitaxel formulated as albumin-bound particles to rats during pregnancy, on gestation days 7 to 17 at doses of 6 mg/m2 (approximately 2% of the daily maximum recommended human dose on a mg/m2 basis) caused embryofetal toxicities, as indicated by intrauterine mortality, increased resorptions (up to 5-fold), reduced numbers of litters and live fetuses, reduction in fetal body weight and increase in fetal anomalies. Fetal anomalies included soft tissue and skeletal malformations, such as eye bulge, folded retina, microphthalmia, and dilation of brain ventricles. A lower incidence of soft tissue and skeletal malformations were also exhibited at 3 mg/m2 (approximately 1% of the daily maximum recommended human dose on a mg/m2 basis).
Pregnancy Category (AUS): D
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Paclitaxel in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Paclitaxel during labor and delivery.
Nursing Mothers
- It is not known whether paclitaxel is excreted in human milk. Paclitaxel and/or its metabolites were excreted into the milk of lactating rats. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants, a decision should be made to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- The safety and effectiveness of paclitaxel in pediatric patients have not been evaluated.
Geriatic Use
- Of the 229 patients in the randomized study who received paclitaxel for the treatment of metastatic breast cancer, 13% were at least 65 years of age and < 2% were 75 years or older. No toxicities occurred notably more frequently among patients who received paclitaxel.
- Of the 514 patients in the randomized study who received paclitaxel and carboplatin for the first-line treatment of non-small cell lung cancer, 31% were 65 years or older and 3.5% were 75 years or older. Myelosuppression, peripheral neuropathy, and arthralgia were more frequent in patients 65 years or older compared to patients younger than 65 years old. No overall difference in effectiveness, as measured by response rates, was observed between patients 65 years or older compared to patients younger than 65 years old.
- Of the 431 patients in the randomized study who received paclitaxel and gemcitabine for the first-line treatment of pancreatic adenocarcinoma, 41% were 65 years or older and 10% were 75 years or older. No overall differences in effectiveness were observed between patients who were 65 years of age or older and younger patients. Diarrhea, decreased appetite, dehydration and epistaxis were more frequent in patients 65 years or older compared with patients younger than 65 years old. Clinical studies of paclitaxel did not include sufficient number of patients with pancreatic cancer who were 75 years and older to determine whether they respond differently from younger patients.
Gender
There is no FDA guidance on the use of Paclitaxel with respect to specific gender populations.
Race
There is no FDA guidance on the use of Paclitaxel with respect to specific racial populations.
Renal Impairment
Adjustment of the starting paclitaxel dose is not required for patients with mild to moderate renal impairment (estimated creatinine clearance ≥30 to <90 mL/min). There are insufficient data to permit dosage recommendations in patients with severe renal impairment or end stage renal disease (estimated creatinine clearance <30 mL/min).
Hepatic Impairment
- Because the exposure and toxicity of paclitaxel can be increased with hepatic impairment, administration of paclitaxel in patients with hepatic impairment should be performed with caution.
- Patients with hepatic impairment may be at increased risk of toxicity, particularly from myelosuppression; such patients should be closely monitored for development of profound myelosuppression.
- Paclitaxel is not recommended in patients who have total bilirubin >5 x ULN or AST >10 x ULN. In addition, paclitaxel is not recommended in patients with metastatic adenocarcinoma of the pancreas who have moderate to severe hepatic impairment (total bilirubin >1.5 x ULN and AST ≤10 x ULN). The starting dose should be reduced for patients with moderate or severe hepatic impairment.
Females of Reproductive Potential and Males
- Men should be advised not to father a child while receiving paclitaxel.
- Administration of paclitaxel formulated as albumin-bound particles to male rats at 42 mg/m2 on a weekly basis (approximately 16% of the daily maximum recommended human exposure on a body surface area basis) for 11 weeks prior to mating with untreated female rats resulted in significantly reduced fertility accompanied by decreased pregnancy rates and increased loss of embryos in mated females.
Immunocompromised Patients
There is no FDA guidance one the use of Paclitaxel in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Paclitaxel Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Paclitaxel and IV administrations.
Overdosage
There is no known antidote for paclitaxel overdosage. The primary anticipated complications of overdosage would consist of bone marrow suppression, sensory neurotoxicity, and mucositis.
Pharmacology
Mechanism of Action
Paclitaxel is a microtubule inhibitor that promotes the assembly of microtubules from tubulin dimers and stabilizes microtubules by preventing depolymerization. This stability results in the inhibition of the normal dynamic reorganization of the microtubule network that is essential for vital interphase and mitotic cellular functions. Paclitaxel induces abnormal arrays or “bundles” of microtubules throughout the cell cycle and multiple asters of microtubules during mitosis.
Structure
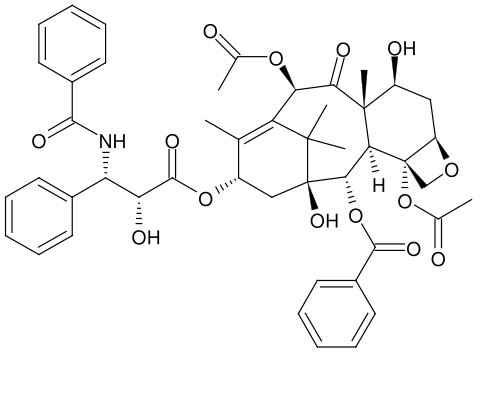
The chemical name for paclitaxel is 5β,20-Epoxy-1,2α,4,7β,10β,13α-hexahydroxytax-11-en-9-one 4,10-diacetate 2-benzoate 13-ester with (2R,3S)-N-benzoyl-3-phenylisoserine.
Paclitaxel has the following structural formula:
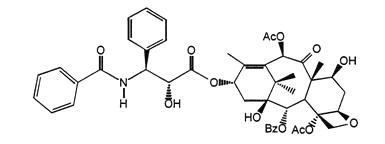
Paclitaxel is a white to off-white crystalline powder with the empirical formula C47H51NO14 and a molecular weight of 853.91.
Pharmacodynamics
There is limited information regarding Paclitaxel Pharmacodynamics in the drug label.
Pharmacokinetics
Absorption
- The pharmacokinetics of total paclitaxel following 30 and 180-minute infusions of paclitaxel at dose levels of 80 to 375 mg/m2 were determined in clinical studies. Paclitaxel plasma concentrations declined in a biphasic manner, the initial rapid decline representing distribution to the peripheral compartment and the slower second phase representing drug elimination.
- The drug exposure (AUCs) was dose proportional over 80 to 300 mg/m2 and the pharmacokinetics of paclitaxel for paclitaxel were independent of the duration of intravenous administration.
Distribution
- Paclitaxel is evenly distributed into blood cells and plasma and is highly bound to plasma proteins (94%). In vitro studies of binding to human serum proteins, using paclitaxel concentrations ranging from 0.1 to 50 µg/mL, indicated that the presence of cimetidine, ranitidine, dexamethasone, or diphenhydramine did not affect protein binding of paclitaxel. The total volume of distribution is approximately 1741 L; the large volume of distribution indicates extensive extravascular distribution and/or tissue binding of paclitaxel.
Metabolism
- In vitro studies with human liver microsomes and tissue slices showed that paclitaxel was metabolized primarily to 6α-hydroxypaclitaxel by CYP2C8; and to two minor metabolites, 3’-p-hydroxypaclitaxel and 6α, 3’-p-dihydroxypaclitaxel, by CYP3A4. In vitro, the metabolism of paclitaxel to 6α-hydroxypaclitaxel was inhibited by a number of agents (ketoconazole, verapamil, diazepam, quinidine, dexamethasone, cyclosporin, teniposide, etoposide, and vincristine), but the concentrations used exceeded those found in vivo following normal therapeutic doses. Testosterone, 17α-ethinyl estradiol, retinoic acid, and quercetin, a specific inhibitor of CYP2C8, also inhibited the formation of 6α-hydroxypaclitaxel in vitro. The pharmacokinetics of paclitaxel may also be altered in vivo as a result of interactions with compounds that are substrates, inducers, or inhibitors of CYP2C8 and/or CYP3A4.
Elimination
- At the clinical dose range of 80 to 300 mg/m2, the mean total clearance of paclitaxel ranges from 13 to 30 L/h/m2, and the mean terminal half-life ranges from 13 to 27 hours.
- After a 30-minute infusion of 260 mg/m2 doses of paclitaxel, the mean values for cumulative urinary recovery of unchanged drug (4%) indicated extensive non-renal clearance. Less than 1% of the total administered dose was excreted in urine as the metabolites 6α-hydroxypaclitaxel and 3’-p-hydroxypaclitaxel.
- Fecal excretion was approximately 20% of the total dose administered.
Nonclinical Toxicology
- The carcinogenic potential of paclitaxel has not been studied.
- Paclitaxel was clastogenic in vitro (chromosome aberrations in human lymphocytes) and in vivo (micronucleus test in mice).
- Administration of paclitaxel formulated as albumin-bound particles to male rats at 42 mg/m2 on a weekly basis (approximately 16% of the daily maximum recommended human exposure on a body surface area basis) for 11 weeks prior to mating with untreated female rats resulted in significantly reduced fertility accompanied by decreased pregnancy rates and increased loss of embryos in mated females.
- A low incidence of skeletal and soft tissue fetal anomalies was also observed at doses of 3 and 12 mg/m2/week in this study (approximately 1 to 5% of the daily maximum recommended human exposure on a mg/m2 basis).
- Testicular atrophy/degeneration was observed in single-dose toxicology studies in rodents administered paclitaxel formulated as albumin-bound particles at doses lower than the recommended human dose; doses were 54 mg/m2 in rodents and 175 mg/m2 in dogs.
Clinical Studies
Metastatic Breast Cancer
- Data from 106 patients accrued in two single arm open label studies and from 460 patients enrolled in a randomized comparative study were available to support the use of paclitaxel in metastatic breast cancer.
Single Arm Open Label Studies
- In one study, paclitaxel was administered as a 30-minute infusion at a dose of 175 mg/m2 to 43 patients with metastatic breast cancer. The second trial utilized a dose of 300 mg/m2 as a 30-minute infusion in 63 patients with metastatic breast cancer. Cycles were administered at 3-week intervals. Objective responses were observed in both studies.
Randomized Comparative Study
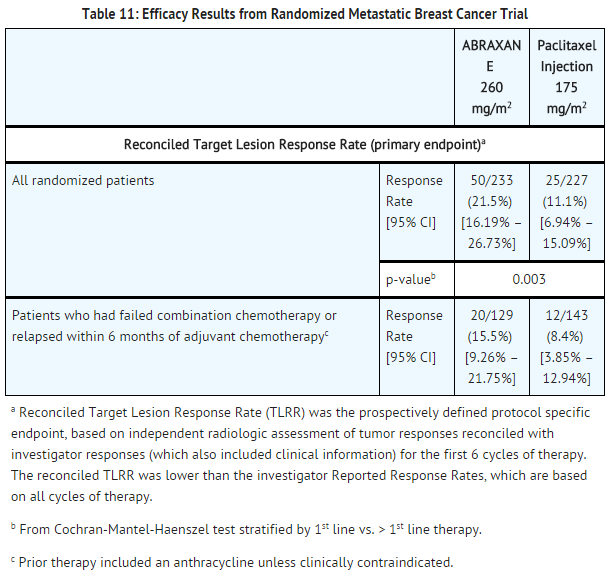
- This multicenter trial was conducted in 460 patients with metastatic breast cancer. Patients were randomized to receive paclitaxel at a dose of 260 mg/m2 given as a 30-minute infusion, or paclitaxel injection at 175 mg/m2 given as a 3-hour infusion. Sixty-four percent of patients had impaired performance status (ECOG 1 or 2) at study entry; 79% had visceral metastases; and 76% had > 3 sites of metastases. Fourteen percent of the patients had not received prior chemotherapy; 27% had received chemotherapy in the adjuvant setting, 40% in the metastatic setting and 19% in both metastatic and adjuvant settings. Fifty-nine percent received study drug as second or greater than second-line therapy. Seventy-seven percent of the patients had been previously exposed to anthracyclines.
- In this trial, patients in the paclitaxel treatment arm had a statistically significantly higher reconciled target lesion response rate (the trial primary endpoint) of 21.5% (95% CI: 16.2% to 26.7%), compared to 11.1% (95% CI: 6.9% to 15.1%) for patients in the paclitaxel injection treatment arm. There was no statistically significant difference in overall survival between the two study arms.
Non-Small Cell Lung Cancer
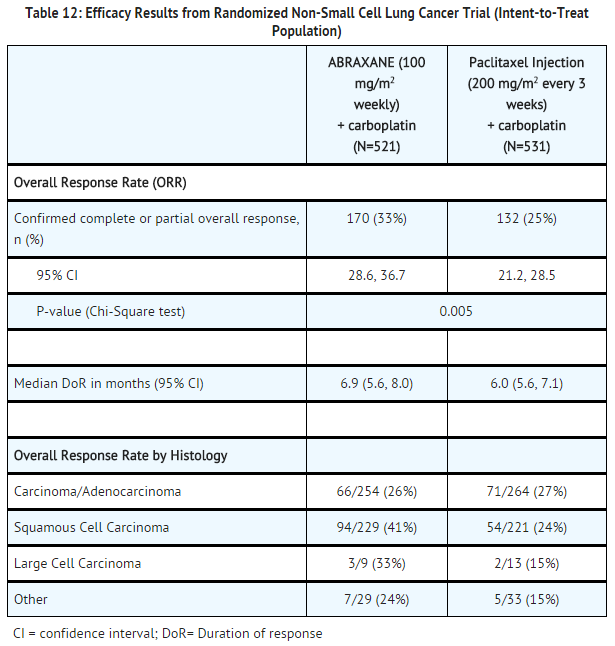
- A multicenter, randomized, open-label study was conducted in 1052 chemonaive patients with Stage IIIb/IV non-small cell lung cancer to compare ABRAXANE in combination with carboplatin to paclitaxel injection in combination with carboplatin as first-line treatment in patients with advanced non-small cell lung cancer. ABRAXANE was administered as an intravenous infusion over 30 minutes at a dose of 100 mg/m2 on Days 1, 8, and 15 of each 21-day cycle. Paclitaxel injection was administered as an intravenous infusion over 3 hours at a dose of 200 mg/m2, following premedication. In both treatment arms carboplatin at a dose of AUC = 6 mg•min/mL was administered intravenously on Day 1 of each 21-day cycle after completion of ABRAXANE/paclitaxel infusion. Treatment was administered until disease progression or development of an unacceptable toxicity. The major efficacy outcome measure was overall response rate as determined by a central independent review committee using RECIST guidelines (Version 1.0).
- In the intent-to-treat (all-randomized) population, the median age was 60 years, 75% were men, 81% were White, 49% had adenocarcinoma, 43% had squamous cell lung cancer, 76% were ECOG PS 1, and 73% were current or former smokers. Patients received a median of 6 cycles of treatment in both study arms.
- Patients in the ABRAXANE/carboplatin arm had a statistically significantly higher overall response rate compared to patients in the paclitaxel injection/carboplatin arm (33% versus 25%). There was no statistically significant difference in overall survival between the two study arms.
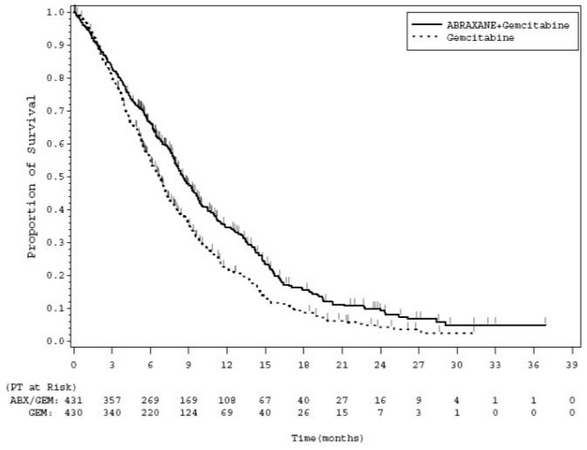
Adenocarcinoma of the Pancreas
- A multicenter, multinational, randomized, open-label study was conducted in 861 patients comparing ABRAXANE plus gemcitabine versus gemcitabine monotherapy as first-line treatment of metastatic adenocarcinoma of the pancreas. Key eligibility criteria were Karnofsky Performance Status (KPS) ≥70, normal bilirubin level, transaminase levels ≤ 2.5 times the upper limit of normal (ULN) or ≤ 5 times the ULN for patients with liver metastasis, no prior cytotoxic chemotherapy in the adjuvant setting or for metastatic disease, no ongoing active infection requiring systemic therapy, and no history of interstitial lung disease. Patients with rapid decline in KPS (≥10%) or serum albumin (≥20%) during the 14 day screening period prior to study randomization were ineligible.
- A total of 861 patients were randomized (1:1) to the ABRAXANE/gemcitabine arm (N=431) or to the gemcitabine arm (N=430). Randomization was stratified by geographic region (Australia, Western Europe, Eastern Europe, or North America), KPS (70 to 80 versus 90 to 100), and presence of liver metastasis (yes versus no). Patients randomized to ABRAXANE/gemcitabine received ABRAXANE 125 mg/m2 as an intravenous infusion over 30-40 minutes followed by gemcitabine 1000 mg/m2 as an intravenous infusion over 30-40 minutes on Days 1, 8, and 15 of each 28-day cycle. Patients randomized to gemcitabine received 1000 mg/m2 as an intravenous infusion over 30-40 minutes weekly for 7 weeks followed by a 1-week rest period in Cycle 1 then as 1000 mg/m2 on Days 1, 8 and 15 of each subsequent 28-day cycle. Patients in both arms received treatment until disease progression or unacceptable toxicity. The major efficacy outcome measure was overall survival (OS). Additional outcome measures were progression-free survival (PFS) and overall response rate (ORR), both assessed by independent, central, blinded radiological review using RECIST (version 1.0).
- In the intent to treat (all randomized) population, the median age was 63 years (range 27-88 years) with 42% ≥ 65 years of age; 58% were men; 93% were White and KPS was 90-100 in 60%. Disease characteristics included 46% of patients with 3 or more metastatic sites; 84% of patients had liver metastasis; and the location of the primary pancreatic lesion was in the head of pancreas (43%), body (31%), or tail (25%).
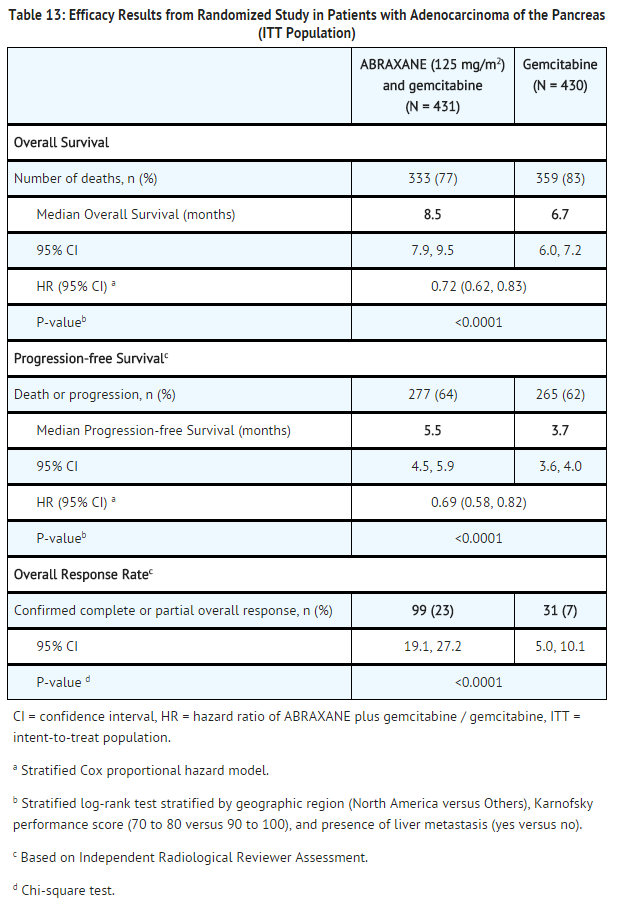
- In exploratory analyses conducted in clinically relevant subgroups with a sufficient number of subjects, the treatment effects on overall survival were similar to that observed in the overall study population.
How Supplied
100 mg of paclitaxel in a single-use vial
- Individually packaged in a carton.
- NDC No.: 68817-134-50
Storage
At 20°C to 25°C (68°F to 77°F).
Images
Drug Images
{{#ask: Page Name::Paclitaxel |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Paclitaxel |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Paclitaxel injection may cause fetal harm. Advise patients to avoid becoming pregnant while receiving this drug. Women of childbearing potential should use effective contraceptives while receiving paclitaxel.
- Advise men not to father a child while receiving paclitaxel.
- Patients must be informed of the risk of low blood cell counts and severe and life-threatening infections and instructed to contact their physician immediately for fever or evidence of infection.
- Patients should be instructed to contact their physician for persistent vomiting, diarrhea, or signs of dehydration.
- Patients must be informed that sensory neuropathy occurs frequently with paclitaxel and patients should advise their physicians of numbness, tingling, pain or weakness involving the extremities.
- Explain to patients that alopecia, fatigue/asthenia, and myalgia/arthralgia occur frequently with paclitaxel.
- Instruct patients to contact their physician for signs of an allergic reaction, which could be severe and sometimes fatal.
- Instruct patients to contact their physician immediately for sudden onset of dry persistent cough, or shortness of breath.
Precautions with Alcohol
Alcohol-Paclitaxel interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Taxol
- Onxol
- Nov-Onxol
- Paclitaxel Novaplus
- Abraxane [2]
Look-Alike Drug Names
There is limited information regarding Paclitaxel Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Peltier, Sandra; Oger, Jean-Michel; Lagarce, Frédéric; Couet, William; Benoît, Jean-Pierre (2006). "Enhanced Oral Paclitaxel Bioavailability After Administration of Paclitaxel-Loaded Lipid Nanocapsules". Pharmaceutical Research. 23 (6): 1243–50. doi:10.1007/s11095-006-0022-2. PMID 16715372.
- ↑ "FDA LABEL: ABRAXANE- paclitaxel injection, powder, lyophilized, for suspension".
{{#subobject:
|Label Page=Paclitaxel |Label Name=Paclitaxel 100mg.jpg
}}