Moxetumomab pasudotox-tdfk
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Zach Leibowitz [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
CAPILLARY LEAK SYNDROME AND HEMOLYTIC UREMIC SYNDROME
See full prescribing information for complete Boxed Warning.
|
Overview
Moxetumomab pasudotox-tdfk is a CD22-directed cytotoxin that is FDA approved for the treatment of adult patients with relapsed or refractory hairy cell leukemia (HCL) who received at least two prior systemic therapies, including treatment with a purine nucleoside analog (PNA). There is a Black Box Warning for this drug as shown here. Common adverse reactions include infusion-related reactions, edema, nausea, fatigue, headache, pyrexia, constipation, anemia, and diarrhea. Most common (≥ 50%) laboratory abnormalities are creatinine increased, ALT increased, hypoalbuminemia, AST increased, hypocalcemia, and hypophosphatemia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Moxetumomab pasudotox-tdfk is indicated for the treatment of adult patients with relapsed or refractory hairy cell leukemia (HCL) who received at least two prior systemic therapies, including treatment with a purine nucleoside analog (PNA).
Limitations of Use
- Moxetumomab pasudotox-tdfk is not recommended in patients with severe renal impairment (CrCl ≤ 29 mL/min).
Dosage
- The recommended dose of moxetumomab pasudotox-tdfk is 0.04 mg/kg administered as a 30-minute intravenous infusion on Days 1, 3, and 5 of each 28-day cycle. Continue moxetumomab pasudotox-tdfk treatment for a maximum of 6 cycles, disease progression, or unacceptable toxicity.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Moxetumomab pasudotox Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding Moxetumomab pasudotox Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness have not been established in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Moxetumomab pasudotox Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding Moxetumomab pasudotox Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
None.
Warnings
|
CAPILLARY LEAK SYNDROME AND HEMOLYTIC UREMIC SYNDROME
See full prescribing information for complete Boxed Warning.
|
Capillary Leak Syndrome (CLS)
- Capillary leak syndrome (CLS), including life-threatening cases, has been reported among patients treated with moxetumomab pasudotox-tdfk and is characterized by hypoalbuminemia, hypotension, symptoms of fluid overload, and hemoconcentration. In the combined safety database of HCL patients treated with moxetumomab pasudotox-tdfk, CLS occurred in 34% (44/129) of patients, including Grade 2 in 23% (30/129), Grade 3 in 1.6% (2/129), and Grade 4 in 2% (3/129).
- Most cases of CLS occurred in the first 8 days (range: 1 to 19) of a treatment cycle, however, cases have also been reported on other days throughout the cycle. The median time to resolution of CLS was 12 days (range: 1 to 53).
- Monitor patient weight and blood pressure prior to each moxetumomab pasudotox-tdfk infusion and as clinically indicated during treatment. Assess patients for signs and symptoms of CLS, including weight gain (increase in 5.5 pounds (2.5 kg) or ≥ 5% from Day 1 of current cycle), hypotension, peripheral edema, shortness of breath or cough, and pulmonary edema and/or serosal effusions. In addition, the following changes in laboratory parameters may help identify CLS: hypoalbuminemia, elevated hematocrit, leukocytosis, and thrombocytosis.
- CLS may be life-threatening or fatal if treatment is delayed. Counsel patients to seek immediate medical attention should signs or symptoms of CLS occur at any time. Patients who develop CLS should receive appropriate supportive measures, including concomitant oral or intravenous corticosteroids, and hospitalization as clinically indicated. Withhold moxetumomab pasudotox-tdfk for Grade 2 CLS until resolution, and permanently discontinue for Grade ≥ 3 CLS.
Hemolytic Uremic Syndrome (HUS)
- Hemolytic Uremic Syndrome (HUS), including life threatening cases, has been reported in patients treated with moxetumomab pasudotox-tdfk and is characterized by the triad of microangiopathic hemolytic anemia, thrombocytopenia, and progressive renal failure. In the combined safety database of HCL patients treated with moxetumomab pasudotox-tdfk, HUS occurred in 7% (9/129) of patients, including Grade 3 in 3% (4/129) and Grade 4 in 0.8% (1/129).
- Most cases of HUS occurred in the first 9 days (range: 1 to 16) of a treatment cycle, however, cases have also been reported on other days throughout the cycle. The median time to resolution of HUS was 11.5 days (range: 2 to 44). All cases resolved, including those who discontinued moxetumomab pasudotox-tdfk.
- Avoid moxetumomab pasudotox-tdfk in patients with prior history of severe thrombotic microangiopathy (TMA) or HUS. Administer prophylactic intravenous fluids before and after moxetumomab pasudotox-tdfk infusions. In Study 1053, patients with a platelet count ≥ 100,000/mm3 received low-dose aspirin on Days 1 through 8 of each 28-day cycle for prophylaxis of thrombosis.
- Monitor blood chemistry and complete blood counts prior to each dose and on Day 8 of each treatment cycle. Monitoring mid-cycle is also recommended. Consider the diagnosis of HUS in patients who develop hemolytic anemia, worsening or sudden onset of thrombocytopenia, increase in creatinine levels, elevation of bilirubin and/or LDH, and have evidence of hemolysis based on peripheral blood smear schistocytes.
- The events of HUS may be life-threatening if treatment is delayed with increased risk of progressive renal failure requiring dialysis. If HUS is suspected initiate appropriate supportive measures, including fluid repletion, hemodynamic monitoring, and consider hospitalization as clinically indicated. Discontinue moxetumomab pasudotox-tdfk in patients with HUS.
Renal Toxicity
- Renal toxicity has been reported in patients treated with moxetumomab pasudotox-tdfk therapy. In the combined safety database of HCL patients treated with moxetumomab pasudotox-tdfk, 26% (34/129) reported adverse events of renal toxicity, including acute kidney injury (2.3%), renal failure (2.3%), renal impairment (1.6%), serum creatinine increased (17%), and proteinuria (8%). Grade 3 acute kidney injury occurred in 1.6% (2/129) of patients. All other events were mild to moderate in severity.
- Based on laboratory findings, during treatment, serum creatinine increased by two or more grades from baseline in 22% (29/129) of patients, including increases of Grade 3 in 1.6% (2/129) of patients. At the end of treatment, serum creatinine levels remained elevated at 1.5- to 3-times the upper limit of normal in 5% of patients. Patients who experience HUS, those ≥ 65 years of age, or those with baseline renal impairment may be at increased risk for worsening of renal function following treatment with moxetumomab pasudotox-tdfk.
- Monitor renal function prior to each infusion of moxetumomab pasudotox-tdfk, and as clinically indicated throughout treatment. Delay moxetumomab pasudotox-tdfk dosing in patients with Grade ≥ 3 elevations in creatinine, or upon worsening from baseline by ≥ 2 grades.
Infusion Related Reactions
- Infusion-related reactions occurred in patients treated with moxetumomab pasudotox-tdfk, and were defined as the occurrence of any one of the following events on the day of study drug infusion: chills, cough, dizziness, dyspnea, feeling hot, flushing, headache, hypertension, hypotension, infusion-related reaction, myalgia, nausea, pyrexia, sinus tachycardia, tachycardia, vomiting, or wheezing. In Study 1053, infusion related reactions occurred in 50% (40/80) of patients. Grade 3 infusion related events as defined, occurred in 11% (9/80) of moxetumomab pasudotox-tdfk-treated patients. The most frequently reported infusion related events were nausea (15%), pyrexia (14%), chills (14%), vomiting (11%), headache (9%), and infusion-related reaction (9%).
- Infusion-related reactions may occur during any cycle of treatment with moxetumomab pasudotox-tdfk. Prior to each dose of moxetumomab pasudotox-tdfk, premedicate with antihistamines and antipyretics. If a severe infusion-related reaction occurs, interrupt the moxetumomab pasudotox-tdfk infusion and institute appropriate medical management. Administer an oral or intravenous corticosteroid approximately 30 minutes before resuming, or before the next moxetumomab pasudotox-tdfk infusion.
Electrolyte Abnormalities
- In the combined safety database of HCL patients treated with moxetumomab pasudotox-tdfk, electrolyte abnormalities occurred in 57% (73/129) of patients with the most common electrolyte abnormality being hypocalcemia occurring in 25% of patients. Grade 3 electrolyte abnormalities occurred in 14% (18/129) of patients and Grade 4 electrolyte abnormalities occurred in 0.8% (1/129) of patients. Electrolyte abnormalities co-occurred in the same treatment cycle with CLS, HUS, fluid retention, or renal toxicity in 37% (48/129) of patients.
- Monitor serum electrolytes prior to each dose and on Day 8 of each treatment cycle. Monitoring mid-cycle is also recommended.
Adverse Reactions
Clinical Trials Experience
- As clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety data described in this section reflect exposure to moxetumomab pasudotox-tdfk in 80 patients with previously treated HCL in Study 1053. Patients received moxetumomab pasudotox-tdfk 0.04 mg/kg as an intravenous infusion over 30 minutes on Days 1, 3, and 5 of each 28‑day cycle for a maximum of 6 cycles or until disease progression or unacceptable toxicity.
- The median duration of treatment with moxetumomab pasudotox-tdfk was 5.7 months (range: 0.9 to 6.7), with a median of 6 treatment cycles started in each patient.
- The most common non-laboratory adverse reactions (≥ 20%) of any grade were infusion-related reactions, edema, nausea, fatigue, headache, pyrexia, constipation, anemia, and diarrhea. The most common Grade 3 or 4 adverse reactions (reported in at least ≥ 5% of patients) were hypertension, febrile neutropenia, and HUS.
- The most common laboratory abnormalities (≥ 20%) of any grade were creatinine increased, ALT increased, hypoalbuminemia, AST increased, hypocalcemia, hypophosphatemia, hemoglobin decreased, neutrophil count decreased, hyponatremia, blood bilirubin increased, hypokalemia, GGT increased, hypomagnesemia, platelet count decreased, hyperuricemia, and alkaline phosphate increased.
- Adverse reactions resulting in permanent discontinuation of moxetumomab pasudotox-tdfk occurred in 15% (12/80) of patients. The most common adverse reaction leading to moxetumomab pasudotox-tdfk discontinuation was HUS (5%). The most common adverse reaction resulting in dose delays, omissions, or interruptions was pyrexia (3.8%).
- Tables 4 and 5 present the frequency category of adverse reactions and key laboratory abnormalities observed in patients with relapsed or refractory HCL treated with moxetumomab pasudotox-tdfk.
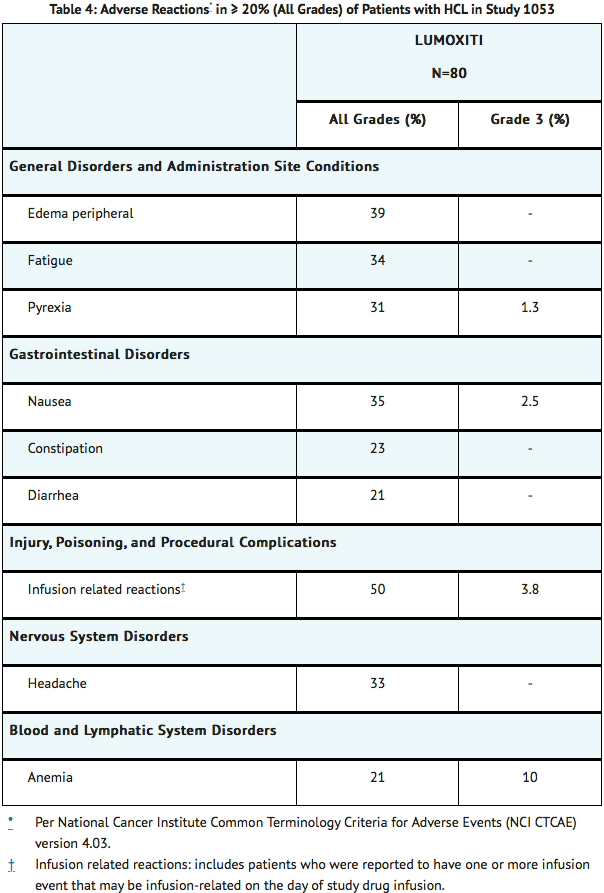
- Fluid retention occurred in 63% (50/80) of patients treated with moxetumomab pasudotox-tdfk in Study 1053, including Grade 3 in 1.3% (1/80) of patients. Fluid retention included all preferred terms of edema peripheral (39%), face edema (14%), abdominal distension (13%), weight increased (8%), pleural effusion (6%), edema (5%), peripheral swelling (5%), localized edema (3.8%), ascites (1.3%), fluid overload (1.3%), fluid retention (1.3%), and pericardial effusion (1.3%). Of the fifty patients with fluid retention, 29% of patients required diuretics.
- Ocular adverse events occurred, including: blurred vision (9%), dry eye (8%), cataracts (5%), ocular discomfort and/or pain (4%), ocular swelling/periorbital edema (4%), conjunctivitis (1.3%), conjunctival hemorrhage (1.3%), and ocular discharge (1.3%).

Immunogenicity
- As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to moxetumomab pasudotox-tdfk in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
- The immunogenicity of moxetumomab pasudotox-tdfk was evaluated using electrochemiluminescent (ECL)-based immunoassay to test for anti-moxetumomab pasudotox-tdfk antibodies (ADA). For patients whose serum tested positive for ADA, a cell-based assay was performed to detect neutralizing antibodies (nAb). In Study 1053, 59% (45/76) of patients tested positive for ADA prior to any treatment with moxetumomab pasudotox-tdfk. Seventy out of 80 subjects tested ADA positive at any point during the study and were subsequently tested for nAb. The results showed that 67 of 70 subjects were nAb-positive. Among these 67 patients who tested nAb-positive, 99% (66/67) had ADA specific to the PE38 binding domain, and 54% (36/67) also had ADA specific to the CD22 binding domain. In 41 out of 73 patients who had baseline and post-baseline ADA results, the median fold increase from baseline (Cycle 1, Day 1) in ADA titer was 3.75- (range: 0 to 240), 54- (range: 0 to 2560), 120- (range: 0 to 1920), and 128- (range: 0 to 2560) fold at Cycles 2, 3, 5, and end-of-treatment, respectively. Patients who tested positive for ADA had decreased systemic moxetumomab pasudotox-tdfk concentrations.
Postmarketing Experience
There is limited information regarding Moxetumomab pasudotox-tdfk Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Moxetumomab pasudotox-tdfk Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Risk Summary
- Based on its mechanism of action and findings in non-pregnant female animals, moxetumomab pasudotox-tdfk is expected to cause maternal and embryo-fetal toxicity when administered to a pregnant woman. There are no available data on moxetumomab pasudotox-tdfk use in pregnant women to inform a drug-associated risk of major birth defects and miscarriage. Animal reproduction or developmental toxicity studies have not been conducted with moxetumomab pasudotox-tdfk. Advise pregnant women of the potential risk to a fetus.
- The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Moxetumomab pasudotox-tdfk in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Moxetumomab pasudotox-tdfk during labor and delivery.
Nursing Mothers
Risk Summary
- No data are available regarding the presence of moxetumomab pasudotox-tdfk in human milk, the effects on the breastfed child, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for moxetumomab pasudotox-tdfk and any potential adverse effects on the breastfed child from moxetumomab pasudotox-tdfk or from the underlying maternal condition.
Pediatric Use
- Safety and effectiveness have not been established in pediatric patients.
Geriatic Use
- In the combined safety database of HCL patients treated with moxetumomab pasudotox-tdfk, 31% (40/129) of patients treated with moxetumomab pasudotox-tdfk were 65 years of age or older and 8% (10/129) were 75 years of age or older. Exploratory analyses across this population suggest a higher incidence of adverse reactions leading to drug discontinuation (23% versus 7%) and renal toxicity (40% versus 20%) for patients 65 years of age or older as compared to those younger than 65 years. Clinical studies of moxetumomab pasudotox-tdfk did not include sufficient numbers of subjects aged 65 and over to determine whether there were differences in efficacy between younger and older patients.
Gender
There is no FDA guidance on the use of Moxetumomab pasudotox-tdfk with respect to specific gender populations.
Race
There is no FDA guidance on the use of Moxetumomab pasudotox-tdfk with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Moxetumomab pasudotox-tdfk in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Moxetumomab pasudotox-tdfk in patients with hepatic impairment.
Females of Reproductive Potential and Males
Contraception
Females
- To avoid potential exposure to the fetus, women of reproductive potential should use effective contraception during treatment with moxetumomab pasudotox-tdfk and for at least 30 days after the last dose is received. Verify the pregnancy status of females of reproductive potential prior to initiating moxetumomab pasudotox-tdfk.
Immunocompromised Patients
There is no FDA guidance one the use of Moxetumomab pasudotox-tdfk in patients who are immunocompromised.
Administration and Monitoring
Administration
Recommended Dosage
- The recommended dose of moxetumomab pasudotox-tdfk is 0.04 mg/kg administered as a 30-minute intravenous infusion on Days 1, 3, and 5 of each 28-day cycle. Continue moxetumomab pasudotox-tdfk treatment for a maximum of 6 cycles, disease progression, or unacceptable toxicity.
Recommended Concomitant Treatment
Hydration
- Intravenously administer 1 L of isotonic solution (e.g., 5% Dextrose Injection, USP and 0.45% or 0.9% Sodium Chloride Injection, USP) over 2‑4 hours before and after each moxetumomab pasudotox-tdfk infusion. Administer 0.5 L to patients under 50 kg.
- Advise all patients to adequately hydrate with up to 3 L (twelve 8‑oz glasses) of oral fluids (e.g., water, milk, or juice) per 24 hours on Days 1 through 8 of each 28‑day cycle. In patients under 50 kg, up to 2 L (eight 8‑oz glasses) per 24 hours is recommended.
- Monitor fluid balance and serum electrolytes to avoid fluid overload and/or electrolyte abnormalities.
Thromboprophylaxis
- Consider low-dose aspirin on Days 1 through 8 of each 28-day cycle.
- Monitor for signs and symptoms of thrombosis.
Premedication
- Premedicate 30‑90 minutes prior to each moxetumomab pasudotox-tdfk infusion with:
- An antihistamine (e.g., hydroxyzine or diphenhydramine)
- Acetaminophen antipyretic
- A histamine‑2 receptor antagonist (e.g., ranitidine, famotidine, or cimetidine)
- If a severe infusion-related reaction occurs, interrupt the moxetumomab pasudotox-tdfk infusion and institute appropriate medical management. Administer an oral or intravenous corticosteroid approximately 30 minutes before resuming, and before each moxetumomab pasudotox-tdfk infusion thereafter.
Post-infusion Medication
- Consider oral antihistamines and antipyretics for up to 24 hours following moxetumomab pasudotox-tdfk infusions.
- An oral corticosteroid (e.g., 4 mg dexamethasone) is recommended to decrease nausea and vomiting.
- Maintain adequate oral fluid intake.
Monitoring to Assess Safety
- Manage adverse reactions by withholding and/or discontinuing moxetumomab pasudotox-tdfk as described below.
- Identify Capillary Leak Syndrome (CLS) and Hemolytic Uremic Syndrome (HUS) based on clinical presentation (see Table 1).

- Adverse reactions graded by the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.03.
Capillary Leak Syndrome (CLS).
- Patients who experience Grade 2 or higher CLS should receive appropriate supportive measures, including treatment with oral or intravenous corticosteroids, with monitoring of weight, albumin levels, and blood pressure until resolution.

- Per National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.03.
Hemolytic Uremic Syndrome (HUS)
- Discontinue moxetumomab pasudotox-tdfk in patients with HUS. Treat with appropriate supportive measures and fluid replacement, with monitoring of blood chemistry, complete blood counts, and renal function until resolution.
Increased Creatinine
- For patients with baseline serum creatinine within normal limits, delay dosing for Grade 2 or higher creatinine increases (greater than 1.5‑times baseline or the upper limit of normal). Resume moxetumomab pasudotox-tdfk upon recovery to Grade 1 (1‑ to 1.5‑times baseline, or between the upper limit of normal and 1.5‑times the upper limit of normal).
- For patients with baseline serum creatinine of Grades 1 or 2, delay dosing for creatinine increases to Grade 3 or higher (greater than 3‑times baseline or the upper limit of normal). Resume moxetumomab pasudotox-tdfk upon recovery to baseline grade or lower.
Instructions for Reconstitution, Dilution, and Administration
- Moxetumomab pasudotox-tdfk must be reconstituted and diluted by a healthcare provider using aseptic technique. Refer to the Healthcare Provider Instructions for Use for moxetumomab pasudotox-tdfk for full reconstitution, dilution, and administration information.
Step 1: Calculate Dose
- Calculate the dose (mg) and the number of moxetumomab pasudotox-tdfk vials (1 mg/vial) to be reconstituted. The final concentration of the reconstituted moxetumomab pasudotox-tdfk solution is 1 mg/mL.
- DO NOT round down for partial vials.
- Individualize dosing based on the patient's actual body weight prior to the first dose of the first treatment cycle.
- A change in dose should only be made between cycles when a change in weight of greater than 10% is observed from the weight used to calculate the first dose of the first treatment cycle. No change in dose should be made during a particular cycle.
Step 2: Reconstitution
Reconstitute moxetumomab pasudotox-tdfk vials with Sterile Water for Injection, USP only.
- Reconstitute each moxetumomab pasudotox-tdfk (1 mg/vial) with 1.1 mL Sterile Water for Injection, USP. The resulting 1 mg/mL solution allows a withdrawal volume of 1 mL.
- Direct the Sterile Water for Injection, USP along the walls of the vial and not directly at the lyophilized cake or powder.
- DO NOT reconstitute moxetumomab pasudotox-tdfk vials with the IV Solution Stabilizer.
- Gently swirl the vial until completely dissolved. Invert the vial to ensure all cake or powder in the vial is dissolved. Do not shake.
- Visually inspect that the reconstituted solution is clear to slightly opalescent, colorless to slightly yellow, and free from visible particles. Do not use if solution is cloudy, discolored, or contains any particles.
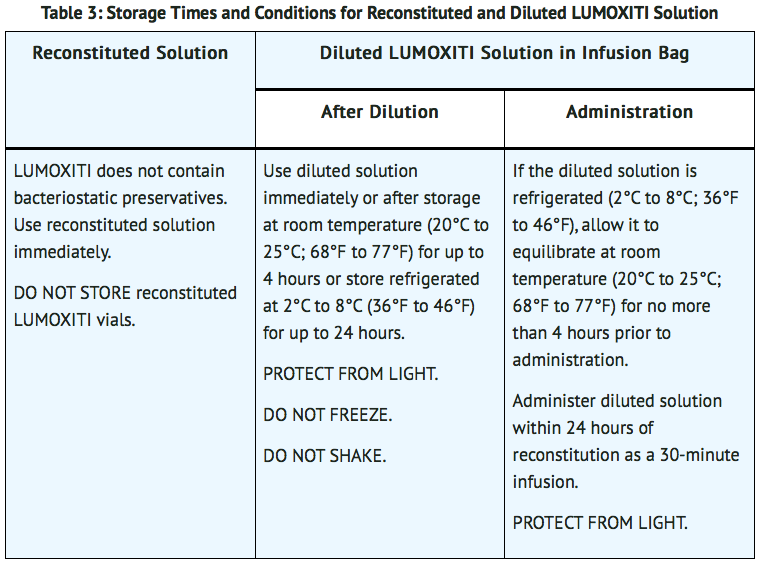
- Use reconstituted solution immediately. Do not store reconstituted moxetumomab pasudotox-tdfk vials. See Table 3 for storage times and conditions for the reconstituted solution.
Step 3: Dilution
Add the IV Solution Stabilizer to the infusion bag prior to adding moxetumomab pasudotox-tdfk solution to the infusion bag. Vial of IV Solution Stabilizer is packaged separately.
- Obtain a 50 mL 0.9% Sodium Chloride Injection, USP infusion bag.
- Add 1 mL IV Solution Stabilizer to the infusion bag containing 50 mL 0.9% Sodium Chloride Injection, USP.
- Only one vial of IV Solution Stabilizer should be used per administration of moxetumomab pasudotox-tdfk.
- Gently invert the bag to mix the solution. Do not shake.
- Withdraw the required volume (calculated from Step 1) of moxetumomab pasudotox-tdfk solution from the reconstituted vial(s).
- Inject moxetumomab pasudotox-tdfk into the infusion bag containing 50 mL 0.9% Sodium Chloride Injection, USP and 1 mL IV Solution Stabilizer.
- Gently invert the bag to mix the solution. Do not shake.
- Discard any partially used or empty vials of moxetumomab pasudotox-tdfk and IV Solution Stabilizer.
- See Table 3 for storage times and conditions for the diluted solution.
Step 4: Administration Instructions
For intravenous infusion only.
- Administer the diluted solution intravenously over 30 minutes.
- Do not mix moxetumomab pasudotox-tdfk, or administer as an infusion with other medicinal products.
- After the infusion, flush the intravenous administration line with of 0.9% Sodium Chloride Injection, USP at the same rate as the infusion. This ensures that the full moxetumomab pasudotox-tdfk dose is delivered.
Monitoring
There is limited information regarding Moxetumomab pasudotox-tdfk Monitoring in the drug label.
IV Compatibility
- Moxetumomab pasudotox is indicated for intravenous use.
Overdosage
There is limited information regarding Moxetumomab pasudotox-tdfk overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
- Moxetumomab pasudotox-tdfk is a CD22-directed cytotoxin. Moxetumomab pasudotox-tdfk binds CD22 on the cell surface of B-cells and is internalized. Moxetumomab pasudotox-tdfk internalization results in ADP-ribosylation of elongation factor 2, inhibition of protein synthesis, and apoptotic cell death.
Structure
There is limited information regarding Moxetumomab pasudotox-tdfk Structure in the drug label.
Pharmacodynamics
- The presence of moxetumomab pasudotox-tdfk may interfere with detection of cellular CD22, therefore, total peripheral blood B-cell counts (including normal B cells and HCL cells) were quantified using a standard assay for CD19+ B cells as a surrogate. In patients with HCL, treatment with moxetumomab pasudotox-tdfk at the approved recommended dosage resulted in a reduction of circulating CD19+ B cells. The circulating CD19+ B cells on Day 8 were reduced by 89% from baseline following the first three infusions of moxetumomab pasudotox-tdfk. B cell reduction was sustained for at least 1-month post-treatment.
- Total counts of CD3+ T cells, CD4+ T cells, CD8+ T cells, and CD16+/CD56 Natural Killer cells and quantitative immunoglobulin (Ig) A, G, and M levels were evaluated throughout the course of treatment. On Day 8, median cell counts were reduced from baseline for the following populations: CD3+ T cells (-21%), CD4+ T cells (-20%), CD8+ T cells (-23%), and CD16+/CD56 Natural Killer cells (-47%). All monitored cell counts returned to, or were elevated above baseline levels on Day 29 and thereafter. At baseline, the median IgA, IgG, and IgM levels were 107 mg/dL (11-260), 834 mg/dL (387-3003), and 42 mg/dL (6-380), respectively, and remained generally unchanged at the end of treatment.
Pharmacokinetics
- The pharmacokinetics (PK) of moxetumomab pasudotox-tdfk was studied in patients with HCL at doses ranging from 0.005 to 0.05 mg/kg (about 0.1 to 1.3 times the approved recommended dosage) administered intravenously over 30 minutes on Days 1, 3, and 5 of a 28-day cycle. Moxetumomab pasudotox-tdfk concentrations increased dose-proportionally over the studied dose range. The mean steady state moxetumomab pasudotox-tdfk exposures at the approved recommended dosages were 379 ng/mL (range: 20 to 862; SD: 262) for Cmax and 626 ng·hour/mL (range: 5 to 1960; SD: 610) for AUC0-last. No systemic accumulation of moxetumomab pasudotox-tdfk was observed. Baseline CD19+ B cells were evaluated for association with the PK exposure and higher PK exposures were significantly associated with low baseline CD19+ counts (p < 0.001).
Distribution
- The population PK model estimated mean volume of distribution of moxetumomab pasudotox-tdfk was 6.5 L (SD 2.4).
Elimination
- The mean elimination half-life of moxetumomab pasudotox-tdfk was 1.4 hours (range: 0.8 to 1.8; SD: 0.35). The population PK model estimated mean systemic clearance of moxetumomab pasudotox-tdfk after the first dose of the first cycle was 25 L/hour (SD: 29.0) and after subsequent dosing was 4 L/hour (SD: 4.4).
Metabolism
- The metabolic pathway of moxetumomab pasudotox-tdfk in humans is unknown, however, other protein therapeutics generally undergo proteolytic degradation into small peptides and amino acids via catabolic pathways.
Specific Populations
- No clinically significant differences in the pharmacokinetics of moxetumomab pasudotox-tdfk were observed for age (36 to 84 years), sex, race (White and non-White), body weight (42 to 152 kg), mild hepatic impairment (total bilirubin ≤ upper limit of normal [ULN] and AST > ULN, or total bilirubin > 1 to 1.5 times ULN and any AST), mild renal impairment (CLcr 60-89 mL/min; n=40), or moderate renal impairment (CLcr 30-59 mL/min; n=4) based on population PK analysis.
- The pharmacokinetics of moxetumomab pasudotox-tdfk in patients with moderate to severe hepatic impairment (total bilirubin > 1.5 ULN) or severe renal impairment (CrCl ≤ 29 mL/min) is unknown.
Anti-Product Antibody Formation Affecting PK
- In patients who were ADA-positive with high titers, the presence of ADA post-baseline was associated with statistically significant (p < 0.05) lower PK exposure (Cmax) at later cycles (Cycle 3 and beyond).
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- No studies have been conducted to assess the carcinogenic or genotoxic potential of moxetumomab pasudotox-tdfk. Animal fertility studies have not been conducted with moxetumomab pasudotox-tdfk.
Animal Toxicology and/or Pharmacology
- At a human equivalent dose > 3 times the recommended dose, degeneration of heart tissue was observed in cynomolgus monkeys. At a human equivalent dose > 10 times the recommended dose, gliosis in the brain, axonal degeneration in the spinal cord, and body tremors were observed in cynomolgus monkeys.
Clinical Studies
- The efficacy of moxetumomab pasudotox-tdfk was based upon Study 1053 titled “A Pivotal Multicenter Trial of Moxetumomab Pasudotox in Relapsed/Refractory Hairy Cell Leukemia” (NCT01829711). Study 1053 was conducted in patients with histologically confirmed HCL or HCL variant with a need for therapy based on presence of cytopenias or splenomegaly and who had received prior treatment with at least 2 systemic therapies, including 1 purine nucleoside analog (PNA). Eligible patients had serum creatinine ≤ 1.5 mg/dL or creatinine clearance ≥ 60 mL/min as estimated by the Cockcroft Gault equation.
- A total of 80 patients were enrolled; 77 with classic HCL and 3 with HCL variant. The median age was 60 years (range: 34 to 84) years, 79% were male, and 94% were Caucasian. At baseline, 98% of patients had an ECOG performance status of 0 or 1. The median number of prior treatments was 3 (range: 2 to 11); all patients received prior PNA therapy, including 29% in combination with rituximab. The most common other prior treatment regimens were rituximab monotherapy (51%), interferon-alpha (25%), and a BRAF inhibitor (18%). At baseline, 33% (26/80) of patients had low hemoglobin (< 10 g/dL), 68% (54/80) of patients had neutropenia (< 1000/mm3), and 84% (67/80) patients had baseline platelet counts < 100,000/mm3. About 35% of patients had enlarged spleens (≥ 14 cm, assessed by BICR) at baseline.
- Patients received moxetumomab pasudotox-tdfk 0.04 mg/kg as an intravenous infusion over 30 minutes on Days 1, 3, and 5 of each 28‑day cycle for a maximum of 6 cycles or until documentation of complete response (CR), disease progression, or unacceptable toxicity. The median duration of follow-up was 16.7 months (range: 2 to 49). An independent review committee (IRC) performed efficacy evaluations using blood, bone marrow, and imaging criteria adapted from previous HCL studies and consensus guidelines.
- Efficacy of moxetumomab pasudotox-tdfk in HCL was evaluated by the IRC-assessed rate of durable CR, as confirmed by maintenance of hematologic remission (hemoglobin ≥ 11 g/dL, neutrophils ≥ 1500/mm3, and platelets ≥ 100,000/mm3 without transfusions or growth factor for at least 4 weeks) more than 180 days after IRC-assessed CR. The IRC-assessed durable CR rate was 30% (24/80 patients; 95% CI: 20, 41).
- Additional efficacy outcome measures included overall response rate (ORR), CR, and duration of response (see Table 6).
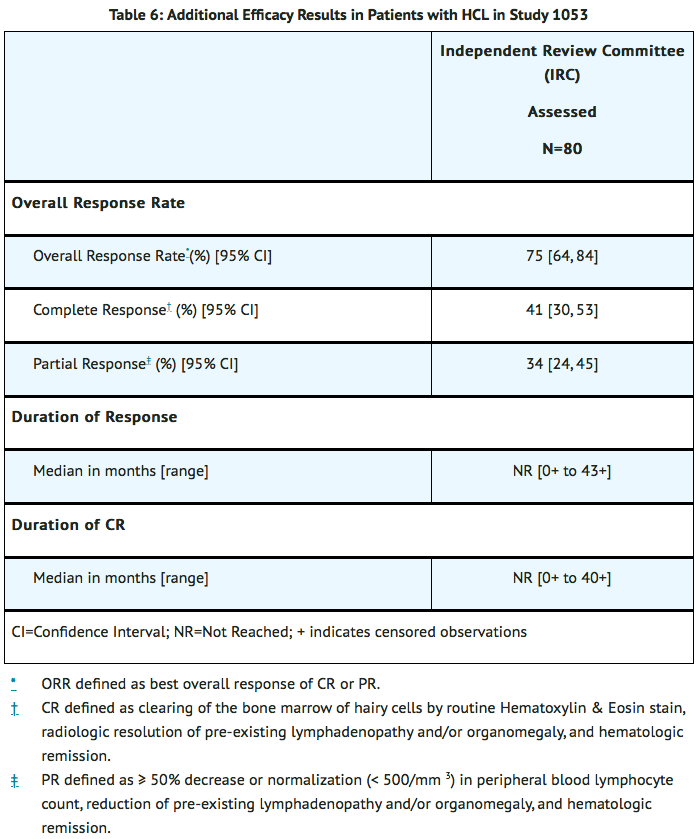
- The median time to ORR and CR was 5.7 months (range: 1.8 to 12.9) and 5.9 months (range 1.8 to 13.2), respectively. Sixty-four patients (80%) had normalization of hematologic parameters and achieved hematologic remission, with a median time to hematologic remission of 1.1 months (range: 0.2 to 13) and with a median duration of hematologic remission not reached (range: 0.3 to 48.2+).
How Supplied
How Supplied
- Moxetumomab pasudotox-tdfk for injection is supplied as a sterile, preservative-free, white to off-white lyophilized cake or powder in a 1 mg single-dose vial. Each carton (NDC 0310-4700-01) contains one single-dose vial.
- IV Solution Stabilizer is supplied as a sterile, preservative-free, colorless to slightly yellow, clear solution free from visible particles in a 1 mL single-dose vial. The IV Solution Stabilizer is packaged separately from moxetumomab pasudotox-tdfk. Each carton (NDC 0310-4715-11) contains one single-dose vial. Do not use the IV Solution Stabilizer to reconstitute moxetumomab pasudotox-tdfk.
- Only one vial of IV Solution Stabilizer should be used per administration of moxetumomab pasudotox-tdfk.
Storage
- Refrigerate moxetumomab pasudotox-tdfk and IV Solution Stabilizer at 2°C to 8°C (36°F to 46°F), in original carton to protect from light. Do not freeze. Do not shake.
Images
Drug Images
{{#ask: Page Name::Moxetumomab pasudotox-tdfk |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Moxetumomab pasudotox-tdfk |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Capillary Leak Syndrome
- Advise patients on the risk of developing capillary leak syndrome. Advise patients to immediately report any symptoms suggestive of capillary leak syndrome, such as difficulty breathing, rapid weight gain, hypotension, or swelling of their arms, legs, and/or face to their healthcare provider for further evaluation.
Hemolytic Uremic Syndrome
- Advise patients on the risk of developing hemolytic uremic syndrome. Advise patients on the importance of maintaining high fluid intake, and the need for frequent monitoring of blood chemistry values.
Renal Toxicity
- Inform patients that taking moxetumomab pasudotox-tdfk may cause decreased renal function. Advise patients to report any changes to urine output to their healthcare provider for further evaluation.
Infusion Related Reactions
- Advise patients to contact their healthcare provider immediately for signs or symptoms of infusion-related reactions.
Electrolyte Abnormalities
- Advise patients to report symptoms of electrolyte abnormalities (e.g., muscle cramping, paresthesias, irregular or fast heartbeat, nausea, seizures) to their healthcare provider immediately.
Medication Guide

Instructions for Use

Precautions with Alcohol
Alcohol-Moxetumomab pasudotox-tdfk interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Moxetumomab pasudotox-tdfk Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.