Maraviroc description
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
Description
SELZENTRY (maraviroc) is a selective, slowly reversible, small molecule antagonist of the interaction between human CCR5 and HIV-1 gp120. Blocking this interaction prevents CCR5-tropic HIV-1 entry into cells.
SELZENTRY is available as film-coated tablets for oral administration containing either 150 or 300 mg of maraviroc and the following inactive ingredients: microcrystalline cellulose, dibasic calcium phosphate (anhydrous), sodium starch glycolate, and magnesium stearate. The film coat [Opadry® II Blue (85G20583)] contains FD&C blue #2 aluminum lake, soya lecithin, polyethylene glycol (macrogol 3350), polyvinyl alcohol, talc and titanium dioxide.
Maraviroc is chemically described as 4,4-difluoro-N-{(1S)-3-[exo-3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]oct-8-yl]-1-phenylpropyl}cyclohexanecarboxamide.
The molecular formula is C29H41F2N5O and the structural formula is:
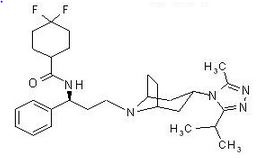 |
Maraviroc is a white to pale colored powder with a molecular weight of 513.67. It is highly soluble across the physiological pH range (pH 1.0 to 7.5).[1]
References
Adapted from the FDA Package Insert.