Maraviroc clinical studies
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
Clinical Studies
The clinical efficacy and safety of SELZENTRY is derived from analyses of data from three ongoing studies in adult subjects infected with CCR5-tropic HIV-1: A4001027 and A4001028, in antiretroviral treatment-experienced adult subjects and A4001026 in treatment-naïve subjects. These studies are supported by a 48-week study in antiretroviral treatment-experienced adult subjects infected with dual/mixed-tropic HIV-1, A4001029.
Studies in CCR5-tropic, Treatment-Experienced Subjects
Studies A4001027 and A4001028 are ongoing, double-blind, randomized, placebo-controlled, multicenter studies in subjects infected with CCR5-tropic HIV-1. Subjects were required to have an HIV-1 RNA of greater than 5,000 copies/mL despite at least 6 months of prior therapy with at least one agent from three of the four antiretroviral drug classes [≥1 nucleoside reverse transcriptase inhibitors (NRTI), ≥1 non-nucleoside reverse transcriptase inhibitors (NNRTI), ≥2 protease inhibitors (PI), and/or enfuvirtide] or documented resistance to at least one member of each class. All subjects received an optimized background regimen consisting of 3 to 6 antiretroviral agents (excluding low-dose ritonavir) selected on the basis of the subject's prior treatment history and baseline genotypic and phenotypic viral resistance measurements. In addition to the optimized background regimen, subjects were then randomized in a 2:2:1 ratio to maraviroc 300 mg once daily, maraviroc 300 mg twice daily, or placebo. Doses were adjusted based on background therapy as described in Dosing and Administration, Table 1.
In the pooled analysis for A4001027 and A4001028, the demographics and baseline characteristics of the treatment groups were comparable (Table 12). Of the 1043 subjects with a CCR5 tropism result at screening, 7.6% had a dual/mixed tropism result at the baseline visit 4 to 6 weeks later. This illustrates the background change from CCR5 to dual/mixed tropism result over time in this treatment-experienced population, prior to a change in antiretroviral regimen or administration of a CCR5 co-receptor antagonist.
 |
The week 48 results for the pooled Studies A4001027 and A4001028 are shown in Table 13.
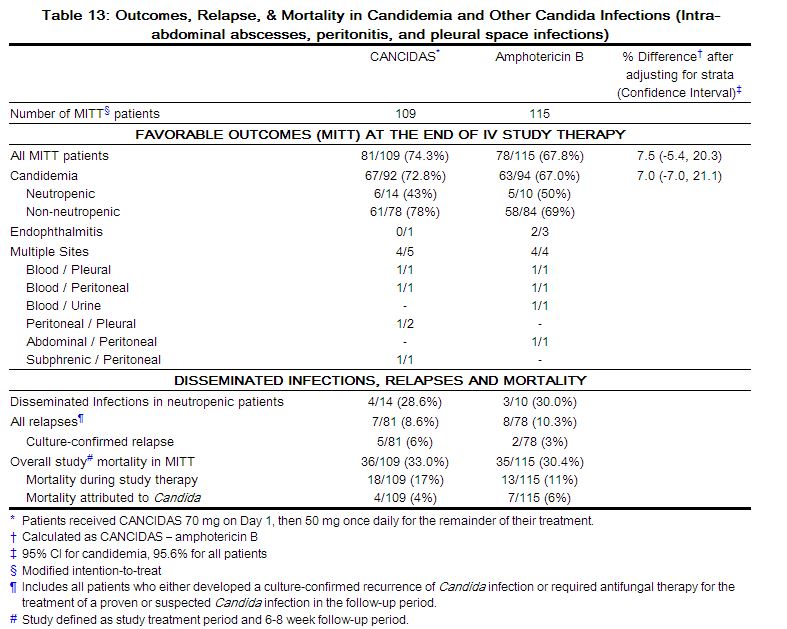 |
After 48 weeks of therapy, the proportion of subjects with HIV-1 RNA <400 copies/mL receiving maraviroc compared to placebo was 56% and 22%, respectively. The mean changes in plasma HIV-1 RNA from baseline to week 48 were –1.84 log10 copies/mL for subjects receiving maraviroc + OBT compared to –0.78 log10 copies/mL for subjects receiving OBT only. The mean increase in CD4+ counts was higher on maraviroc twice daily + OBT (124 cells/mm3) than on placebo + OBT (60 cells/mm3 ).
Study in Dual/Mixed-tropic, Treatment-Experienced Subjects
Study A4001029 was an exploratory, randomized, double-blind, multicenter trial to determine the safety and efficacy of maraviroc in subjects infected with dual/mixed co-receptor tropic HIV-1. The inclusion/exclusion criteria were similar to those for Studies A4001027 and A4001028 above and the subjects were randomized in a 1:1:1 ratio to SELZENTRY once daily, SELZENTRY twice daily, or placebo. No increased risk of infection or HIV disease progression was observed in the subjects who received SELZENTRY. SELZENTRY use was not associated with a significant decrease in HIV-1 RNA compared to placebo in these subjects and no adverse effect on CD4 count was noted.
Study in Treatment-Naïve Subjects
Study A4001026 is an ongoing, randomized, double-blind, multicenter study in subjects infected with CCR5-tropic HIV-1 classified by the original Trofile® tropism assay. Subjects were required to have plasma HIV-1 RNA ≥2000 copies/mL and could not have: 1) previously received any antiretroviral therapy for >14 days, 2) an active or recent opportunistic infection or a suspected primary HIV-1 infection, or 3) phenotypic or genotypic resistance to zidovudine, lamivudine, or efavirenz. Subjects were randomized in a 1:1:1 ratio to maraviroc 300 mg once daily, maraviroc 300 mg twice daily, or efavirenz 600 mg once daily, each in combination with zidovudine/lamivudine. The efficacy and safety of SELZENTRY are based on the comparison of SELZENTRY twice daily versus efavirenz. In a pre-planned interim analysis at 16 weeks, the maraviroc 300mg once per day treatment arm failed to meet the pre-specified criteria for demonstrating non-inferiority and was discontinued.
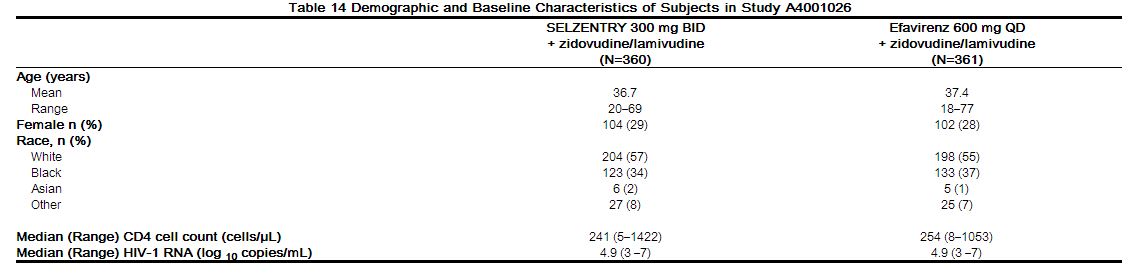
The demographic and baseline characteristics of the maraviroc and efavirenz treatment groups were comparable (Table 14). Subjects were stratified by screening HIV-1 RNA levels and by geographic region. The median CD4 cell counts and mean HIV-1 RNA at baseline were similar for both treatment groups.
 |
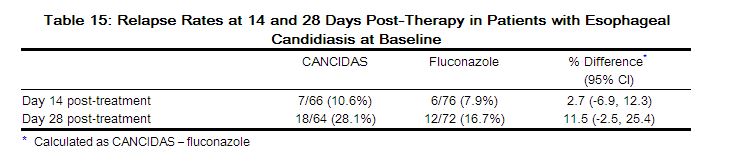
The treatment outcomes at 96 weeks for study A4001026 are shown in Table 15. Treatment outcomes are based on reanalysis of the screening samples using a more sensitive tropism assay, Enhanced sensitivity Trofile® HIV tropism assay, which became available after the week 48 analysis, approximately 15% of the subjects identified as CCR5-tropic in the original analysis had Dual/Mixed- or CXCR4-tropic virus. Screening with enhanced sensitivity version of the Trofile® tropism assay reduced the number of maraviroc virologic failures with CXCR4- or Dual/Mixed-tropic virus at failure to 12 compared to 24 when screening with the original Trofile® HIV tropism assay.
 |
The median increase from baseline in CD4+ cell counts at week 96 was 184 cells/mm3 for the SELZENTRY arm compared to 155 cells/mm3 for the efavirenz arm. [1]
References
Adapted from the FDA Package Insert.