Lumacaftor and ivacaftor
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vishal Devarkonda, M.B.B.S[2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Lumacaftor and ivacaftor is a combination of two cystic fibrosis transmembrane conductance regulators that is FDA approved for the treatment of of cystic fibrosis (CF) in patients age 6 years and older who are homozygous for the F508del mutation in the CFTR gene. If the patient's genotype is unknown, an FDA-cleared CF mutation test should be used to detect the presence of the F508del mutation on both alleles of the CFTR gene. Common adverse reactions include dyspnea, nasopharyngitis, nausea, diarrhea, upper respiratory tract infection, fatigue, and rash.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Lumacaftor and ivacaftor is a combination of lumacaftor and ivacaftor indicated for the treatment of cystic fibrosis (CF) in patients age 6 years and older who are homozygous for the F508del mutation in the CFTR gene. If the patient's genotype is unknown, an FDA-cleared CF mutation test should be used to detect the presence of the F508del mutation on both alleles of the CFTR gene.
The efficacy and safety of lumacaftor and ivacaftor have not been established in patients with CF other than those homozygous for the F508del mutation.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of lumacaftor and ivacaftor in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of lumacaftor and ivacaftor in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Lumacaftor and ivacaftor indicated for the treatment of cystic fibrosis (CF) in patients age 6 years and older who are homozygous for the F508del mutation in the CFTR gene.
Dosing Information

- Dosage Adjustment for Patients with Hepatic Impairment
- No dose adjustment is necessary for patients with mild hepatic impairment (Child-Pugh Class A). A dose reduction to 2 tablets in the morning and 1 tablet in the evening is recommended for patients with moderate hepatic impairment (Child-Pugh Class B).
- Studies have not been conducted in patients with severe hepatic impairment (Child-Pugh Class C), but exposure is expected to be higher than in patients with moderate hepatic impairment. Therefore, use with caution at a maximum dose of 1 tablet in the morning and 1 tablet in the evening, or less, in patients with severe hepatic impairment after weighing the risks and benefits of treatment.
- Dosage Adjustment for Patients Taking CYP3A Inhibitors
- No dose adjustment is necessary when CYP3A inhibitors are initiated in patients already taking lumacaftor and ivacaftor. However, when initiating lumacaftor and ivacaftor in patients currently taking strong CYP3A inhibitors (e.g., itraconazole), reduce lumacaftor and ivacaftor dose to 1 tablet daily for the first week of treatment. Following this period, continue with the recommended daily dose.
- If lumacaftor and ivacaftor is interrupted for more than 1 week and then re-initiated while taking strong CYP3A inhibitors, patients should reduce lumacaftor and ivacaftor dose to 1 tablet daily for the first week of treatment re-initiation. Following this period, continue with the recommended daily dose.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of lumacaftor and ivacaftor in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of lumacaftor and ivacaftor in pediatric patients.
Contraindications
There is limited information regarding Lumacaftor and ivacaftor Contraindications in the drug label.
Warnings
- Use in Patients with Advanced Liver Disease
- Worsening of liver function, including hepatic encephalopathy, in patients with advanced liver disease has been reported in some patients with CF while receiving lumacaftor and ivacaftor.
- Use lumacaftor and ivacaftor with caution in patients with advanced liver disease and only if the benefits are expected to outweigh the risks. If lumacaftor and ivacaftor is used in these patients, they should be closely monitored after the initiation of treatment and the dose should be reduced.
- Liver-related Events
- Serious adverse reactions related to elevated transaminases have been reported in patients with CF receiving lumacaftor and ivacaftor. In some instances, these elevations have been associated with concomitant elevations in total serum bilirubin.
- It is recommended that ALT, AST, and bilirubin be assessed prior to initiating lumacaftor and ivacaftor, every 3 months during the first year of treatment, and annually thereafter.
- For patients with a history of ALT, AST, or bilirubin elevations, more frequent monitoring should be considered. Patients who develop increased ALT, AST, or bilirubin should be closely monitored until the abnormalities resolve.
- Dosing should be interrupted in patients with ALT or AST greater than 5 × upper limit of normal (ULN) when not associated with elevated bilirubin. Dosing should also be interrupted in patients with ALT or AST elevations greater than 3 × ULN when associated with bilirubin elevations greater than 2 × ULN. Following resolution of transaminase elevations, consider the benefits and risks of resuming dosing.
- Respiratory Events
- Respiratory events (e.g., chest discomfort, dyspnea, and respiration abnormal) were observed more commonly in patients during initiation of lumacaftor and ivacaftor compared to those who received placebo.
- Clinical experience in patients with percent predicted FEV1 (ppFEV1) <40 is limited, and additional monitoring of these patients is recommended during initiation of therapy.
- Effect on Blood Pressure
- Increased blood pressure has been observed in some patients treated with lumacaftor and ivacaftor. Blood pressure should be monitored periodically in all patients being treated with lumacaftor and ivacaftor.
- Drug Interactions
- Substrates of CYP3A
- Lumacaftor is a strong inducer of CYP3A. Administration of lumacaftor and ivacaftor may decrease systemic exposure of medicinal products that are substrates of CYP3A, which may decrease therapeutic effect. Co-administration with sensitive CYP3A substrates or CYP3A substrates with a narrow therapeutic index is not recommended.
- Lumacaftor and ivacaftor may substantially decrease hormonal contraceptive exposure, reducing their effectiveness and increasing the incidence of menstruation-associated adverse reactions, e.g., amenorrhea, dysmenorrhea, menorrhagia, menstrual irregularity (27% in women using hormonal contraceptives compared with 3% in women not using hormonal contraceptives). Hormonal contraceptives, including oral, injectable, transdermal, and implantable, should not be relied upon as an effective method of contraception when co-administered with lumacaftor and ivacaftor.
- Strong CYP3A Inducers
- Ivacaftor is a substrate of CYP3A4 and CYP3A5 isoenzymes. Use of lumacaftor and ivacaftor with strong CYP3A inducers, such as rifampin, significantly reduces ivacaftor exposure, which may reduce the therapeutic effectiveness of lumacaftor and Ivacaftor.
- Therefore, co-administration with strong CYP3A inducers (e.g., rifampin, St John's wort [Hypericum perforatum]) is not recommended.
- Substrates of CYP3A
- Cataracts
- Cases of non-congenital lens opacities have been reported in pediatric patients treated with lumacaftor and ivacaftor and ivacaftor. Although other risk factors were present in some cases (such as corticosteroid use and exposure to radiation), a possible risk attributable to ivacaftor cannot be excluded. Baseline and follow-up ophthalmological examinations are recommended in pediatric patients initiating lumacaftor and ivacaftor treatment.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The overall safety profile of lumacaftor and ivacaftor is based on the pooled data from 1108 patients with CF 12 years and older who are homozygous for the F508del mutation in the CFTR gene and who received at least one dose of study drug in 2 double-blind, placebo-controlled, Phase 3 clinical trials, each with 24 weeks of treatment (Trials 1 and 2). Of the 1108 patients, 49% were female and 99% were Caucasian; 369 patients received lumacaftor and ivacaftor every 12 hours and 370 received placebo. Additional safety data in 58 patients with CF aged 6 through 11 years who are homozygous for the F508del-CFTR mutation were obtained from a 24-week, open-label, multicenter Phase 3 safety trial (Trial 3).
The proportion of patients who prematurely discontinued study drug due to adverse events was 5% for patients treated with lumacaftor and ivacaftor and 2% for patients who received placebo.
Serious adverse reactions, whether considered drug-related or not by the investigators, that occurred more frequently in patients treated with lumacaftor and ivacaftor included pneumonia, hemoptysis, cough, increased blood creatine phosphokinase, and transaminase elevations. These occurred in 1% or less of patients.
Table 2 shows adverse reactions occurring in ≥5% of patients with CF ages 12 years and older treated with lumacaftor and ivacaftor who are homozygous for the F508del mutation in the CFTR gene that also occurred at a higher rate than in patients who received placebo in the two double-blind, placebo-controlled trials.
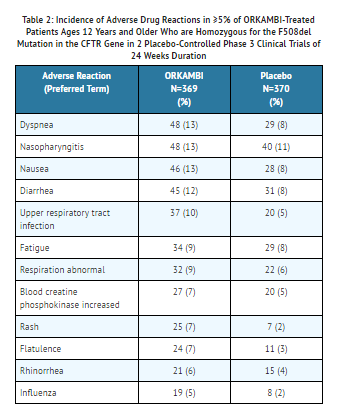
The safety profile from the 24-week, open-label, multicenter Phase 3 safety trial in 58 patients aged 6 through 11 years with CF who are homozygous for the F508del-CFTR mutation (Trial 3) was similar to that observed in Trials 1 and 2.
Additional information on selected adverse reactions from these trials is detailed below.
Description of Selected Adverse Drug Reactions
Liver-related Adverse Reactions
In Trials 1 and 2, the incidence of maximum transaminase (ALT or AST) levels >8, >5, and >3 × ULN elevations was similar between patients treated with lumacaftor and ivacaftor and those who received placebo. Three patients who received lumacaftor and ivacaftor had liver-related serious adverse reactions, including 2 reported as transaminase elevations and 1 as hepatic encephalopathy, compared to none in the placebo group. Of these three, one had elevated transaminases (>3 × ULN) associated with bilirubin elevation >2 × ULN. Following discontinuation or interruption of lumacaftor and ivacaftor, transaminases decreased to <3 × ULN. Among 6 patients with pre-existing cirrhosis and/or portal hypertension who received lumacaftor and ivacaftor, worsening liver function with increased ALT, AST, bilirubin, and hepatic encephalopathy was observed in one patient. The event occurred within 5 days of the start of dosing and resolved following discontinuation of lumacaftor and ivacaftor.
During the 24-week, open-label Phase 3 clinical trial in 58 patients aged 6 through 11 years (Trial 3), the incidence of maximum transaminase (ALT or AST) levels >8, >5, and >3 × ULN was 5%, 9%, and 19%. No patients had an increase in total bilirubin levels > 2 × ULN. Lumacaftor/ivacaftor dosing was maintained or successfully resumed after interruption in all patients with transaminase elevations, except 1 patient who discontinued treatment permanently.
Respiratory Adverse Reactions
In Trials 1 and 2, the incidence of respiratory symptom-related adverse reactions (e.g., chest discomfort, dyspnea, and respiration abnormal) was more common in patients treated with lumacaftor and ivacaftor (22%) compared to patients who received placebo (14%). The incidence of these adverse reactions was more common in patients treated with lumacaftor and ivacaftor with lower pre-treatment FEV1. In patients treated with lumacaftor and ivacaftor, the majority of the events began during the first week of treatment. During the 24-week, open-label Phase 3 clinical trial (Trial 3) in 58 patients aged 6 through 11 years (mean baseline ppFEV1 was 91.4), the incidence of respiratory symptom-related adverse reactions was 3% (2/58).
Menstrual Abnormalities
In Trials 1 and 2, the incidence of combined menstrual abnormality adverse reactions (e.g., amenorrhea, dysmenorrhea, menorrhagia, menstrual irregularity) was more common in female patients treated with lumacaftor and ivacaftor (10%) compared to placebo (2%). These events occurred more frequently in the subset of female patients treated with lumacaftor and ivacaftor who were using hormonal contraceptives (27%) compared to those not using hormonal contraceptives (3%).
Increased Blood Pressure
In Trials 1 and 2, adverse reactions related to increases in blood pressure (e.g., hypertension, blood pressure increased) were reported in 1.1% (4/369) of patients treated with lumacaftor and ivacaftor and in no patients who received placebo.
The proportion of patients who experienced a systolic blood pressure value >140 mmHg or a diastolic blood pressure >90 mmHg on at least two occasions was 3.6% and 2.2% in patients treated with Lumacaftor and ivacaftor, respectively, compared with 1.6% and 0.5% in patients who received placebo.
Postmarketing Experience
There is limited information regarding Lumacaftor and ivacaftor Postmarketing Experience in the drug label.
Drug Interactions
Potential for Other Drugs to Affect Lumacaftor/Ivacaftor
Inhibitors of CYP3A
Co-administration of lumacaftor/ivacaftor with itraconazole, a strong CYP3A inhibitor, did not impact the exposure of lumacaftor, but increased ivacaftor exposure by 4.3-fold. Due to the induction effect of lumacaftor on CYP3A, at steady-state, the net exposure of ivacaftor is not expected to exceed that when given in the absence of lumacaftor at a dose of 150 mg every 12 hours (the approved dose of ivacaftor monotherapy). Therefore, no dose adjustment is necessary when CYP3A inhibitors are initiated in patients currently taking lumacaftor and ivacaftor. However, when initiating lumacaftor and ivacaftor in patients taking strong CYP3A inhibitors, reduce the lumacaftor and ivacaftor dose to 1 tablet daily (lumacaftor 200 mg/ivacaftor 125 mg total daily dose for patients aged 12 years and over; lumacaftor 100 mg/ivacaftor 125 mg total daily dose for patients aged 6 through 11 years) for the first week of treatment to allow for the steady-state induction effect of lumacaftor. Following this period, continue with the recommended daily dose.
Examples of strong CYP3A inhibitors include: ketoconazole, itraconazole, posaconazole, voriconazole, telithromycin, and clarithromycin.
No dose adjustment is recommended when used with moderate or weak CYP3A inhibitors.
Inducers of CYP3A
Co-administration of lumacaftor/ivacaftor with rifampin, a strong CYP3A inducer, had minimal effect on the exposure of lumacaftor, but decreased ivacaftor exposure (AUC) by 57%. This may reduce the effectiveness of lumacaftor and ivacaftor. Therefore, co-administration with strong CYP3A inducers, such as rifampin, rifabutin, phenobarbital, carbamazepine, phenytoin, and St John's wort (Hypericum perforatum), is not recommended.
No dose adjustment is recommended when used with moderate or weak CYP3A inducers.
Potential for Lumacaftor/Ivacaftor to Affect Other Drugs
Lumacaftor is a strong inducer of CYP3A. Co-administration of lumacaftor with ivacaftor, a sensitive CYP3A substrate, decreased ivacaftor exposure by approximately 80%. Administration of lumacaftor and ivacaftor may decrease systemic exposure of medicinal products which are substrates of CYP3A, thereby decreasing the therapeutic effect of the medicinal product.
Co-administration of lumacaftor and ivacaftor is not recommended with sensitive CYP3A substrates or CYP3A substrates with a narrow therapeutic index such as:
- Benzodiazepines: midazolam, triazolam (consider an alternative to these benzodiazepines).
- Immunosuppressants: cyclosporine, everolimus, sirolimus, and tacrolimus (avoid the use of substrates and ivacaftor).
CYP2B6 and CYP2C Substrates
In vitro studies suggest that lumacaftor has the potential to induce CYP2B6, CYP2C8, CYP2C9, and CYP2C19; inhibition of CYP2C8 and CYP2C9 has also been observed in vitro. Additionally, in vitro studies suggest that ivacaftor may inhibit CYP2C9. Therefore, concomitant use of substrates and ivacaftor with CYP2B6, CYP2C8, CYP2C9, and CYP2C19 substrates may alter the exposure of these substrates.
Digoxin and Other P-gp Substrates
Based on in vitro results which showed P-gp inhibition and pregnane-X-receptor (PXR) activation, lumacaftor has the potential to both inhibit and induce P-gp. Additionally, a clinical study with ivacaftor monotherapy showed that ivacaftor is a weak inhibitor of P-gp. Therefore, concomitant use of lumacaftor and ivacaftor with P-gp substrates may alter the exposure of these substrates.
Monitor the serum concentration of digoxin and titrate the digoxin dose to obtain the desired clinical effect.
Anti-allergics and Systemic Corticosteroids
Lumacaftor and ivacaftor may decrease the exposure of montelukast, which may reduce its efficacy. No dose adjustment for montelukast is recommended. Employ appropriate clinical monitoring, as is reasonable, when co-administered with lumacaftor and ivacaftor.
Concomitant use of lumacaftor and ivacaftor may reduce the exposure and effectiveness of prednisone and methylprednisolone. A higher dose of these systemic corticosteroids may be required to obtain the desired clinical effect.
Antibiotics
Concomitant use of lumacaftor and ivacaftor may decrease the exposure of clarithromycin, erythromycin, and telithromycin, which may reduce the effectiveness of these antibiotics. Consider an alternative to these antibiotics, such as ciprofloxacin, azithromycin, and levofloxacin.
Antifungals
Concomitant use of lumacaftor and ivacaftor may reduce the exposure and effectiveness of itraconazole, ketoconazole, posaconazole, and voriconazole. Concomitant use of lumacaftor and ivacaftor with these antifungals is not recommended. Monitor patients closely for breakthrough fungal infections if such drugs are necessary. Consider an alternative such as fluconazole.
Anti-inflammatories
Concomitant use of lumacaftor and ivacaftor may reduce the exposure and effectiveness of ibuprofen. A higher dose of ibuprofen may be required to obtain the desired clinical effect.
Antidepressants
Concomitant use of lumacaftor and ivacaftor may reduce the exposure and effectiveness of citalopram, escitalopram, and sertraline. A higher dose of these antidepressants may be required to obtain the desired clinical effect.
Hormonal Contraceptives
Lumacaftor and ivacaftor may decrease hormonal contraceptive exposure, reducing the effectiveness. Hormonal contraceptives, including oral, injectable, transdermal, and implantable, should not be relied upon as an effective method of contraception when co-administered with lumacaftor and ivacaftor.
Concomitant use of lumacaftor and ivacaftor with hormonal contraceptives increased the menstrual abnormality events. Avoid concomitant use unless the benefit outweighs the risks.
Concomitant use of lumacaftor and ivacaftor may reduce the exposure and effectiveness of repaglinide, and may alter the exposure of sulfonylurea. A dose adjustment may be required to obtain the desired clinical effect. No dose adjustment is recommended for metformin.
Proton Pump Inhibitors, H2 Blockers, Antacids
Lumacaftor and ivacaftor may reduce the exposure and effectiveness of proton pump inhibitors such as omeprazole, esomeprazole, and lansoprazole, and may alter the exposure of ranitidine. A dose adjustment may be required to obtain the desired clinical effect. No dose adjustment is recommended for calcium carbonate antacid.
Lumacaftor and ivacaftor may alter the exposure of warfarin. Monitor the international normalized ratio (INR) when warfarin co-administration with lumacaftor and ivacaftor is required.
Concomitant Drugs That Do Not Need Dose Adjustment
No dosage adjustment of lumacaftor and ivacaftor or concomitant drug is recommended when lumacaftor and ivacaftor is given with the following: azithromycin, aztreonam, budesonide, ceftazidime, cetirizine, ciprofloxacin, colistimethate, colistin, dornase alfa, fluticasone, ipratropium, levofloxacin, pancreatin, pancrelipase, salbutamol, salmeterol, sulfamethoxazole and trimethoprim, tiotropium, and tobramycin. Based on the metabolism and route of elimination, lumacaftor and ivacaftor is not expected to impact the exposure of these drugs.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Risk Summary
There are limited and incomplete human data from clinical trials and postmarketing reports on use of lumacaftor and ivacaftor or its individual components in pregnant women to inform a drug-associated risk. In animal reproduction studies, oral administration of lumacaftor to pregnant rats and rabbits during organogenesis demonstrated no teratogenicity or adverse effects on fetal development at doses that produced maternal exposures up to approximately 8 (rats) and 5 (rabbits) times the exposure at the maximum recommended human dose (MRHD). Oral administration of ivacaftor to pregnant rats and rabbits during organogenesis demonstrated no teratogenicity or adverse effects on fetal development at doses that produced maternal exposures up to approximately 7 (rats) and 45 (rabbits) times the exposure at the MRHD. No adverse developmental effects were observed after oral administration of either lumacaftor or ivacaftor to pregnant rats from organogenesis through lactation at doses that produced maternal exposures approximately 8 and 5 times the exposures at the MRHD, respectively. There are no animal reproduction studies with concomitant administration of lumacaftor and ivacaftor.
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Lumacaftor: In an embryo-fetal development study in pregnant rats dosed during the period of organogenesis from gestation days 7-17, lumacaftor was not teratogenic and did not affect fetal development or survival at exposures up to 8 times the MRHD (on an AUC basis at maternal oral doses up to 2000 mg/kg/day). In an embryo-fetal development study in pregnant rabbits dosed during the period of organogenesis from gestation days 7-19, lumacaftor was not teratogenic and did not affect fetal development or survival at exposures up to 5 times the MRHD (on an AUC basis at maternal oral doses up to 200 mg/kg/day). In a pre- and postnatal development study in pregnant female rats dosed from gestation day 6 through lactation day 20, lumacaftor had no effects on delivery or growth and development of offspring at exposures up to 8 times the MRHD (on an AUC basis at maternal oral doses up to 1000 mg/kg/day). Placental transfer of lumacaftor was observed in pregnant rats and rabbits.
Ivacaftor: In an embryo-fetal development study in pregnant rats dosed during the period of organogenesis from gestation days 7-17, ivacaftor was not teratogenic and did not affect fetal survival at exposures up to 7 times the MRHD (based on summed AUCs for ivacaftor and its metabolites at maternal oral doses up to 200 mg/kg/day). In an embryo-fetal development study in pregnant rabbits dosed during the period of organogenesis from gestation days 7-19, ivacaftor was not teratogenic and did not affect fetal development or survival at exposures up to 45 times the MRHD (on an ivacaftor AUC basis at maternal oral doses up to 100 mg/kg/day). In a pre- and postnatal development study in pregnant female rats dosed from gestation day 7 through lactation day 20, ivacaftor had no effects on delivery or growth and development of offspring at exposures up to 5 times the MRHD (based on summed AUCs for ivacaftor and its metabolites at maternal oral doses up to 100 mg/kg/day). Decreased fetal body weights were observed at a maternally toxic dose that produced exposures 7 times the MRHD (based on summed AUCs for ivacaftor and its metabolites at a maternal oral dose of 200 mg/kg/day). Placental transfer of ivacaftor was observed in pregnant rats and rabbits.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Lumacaftor and ivacaftor in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Lumacaftor and ivacaftor during labor and delivery.
Nursing Mothers
Risk Summary
There is no information regarding the presence of lumacaftor or ivacaftor in human milk, the effects on the breastfed infant, or the effects on milk production. Both lumacaftor and ivacaftor are excreted into the milk of lactating rats; however, due to species-specific differences in lactation physiology, animal lactation data may not reliably predict levels in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for lumacaftor and ivacaftor and any potential adverse effects on the breastfed child from lumacaftor and ivacaftor or from the underlying maternal condition.
Data
Lumacaftor
Lacteal excretion of lumacaftor in rats was demonstrated following a single oral dose (100 mg/kg) of 14C-lumacaftor administered 9 to 11 days postpartum to lactating mothers (dams). Exposure (AUC0-24h) values for lumacaftor in milk were approximately 40% of plasma levels.
Lacteal excretion of ivacaftor in rats was demonstrated following a single oral dose (100 mg/kg) of 14C-ivacaftor administered 9 to 10 days postpartum to lactating mothers (dams). Exposure (AUC0-24h) values for ivacaftor in milk were approximately 1.5 times higher than plasma levels.
Pediatric Use
The efficacy of lumacaftor and ivacaftor in children ages 6 through 11 years is extrapolated from efficacy in patients ages 12 years and older homozygous for the F508del mutation in the CFTR gene with support from population pharmacokinetic analyses showing similar drug exposure levels in patients ages 12 years and older and in children ages 6 through 11 years.
Additional safety data were obtained from a 24-week, open-label, Phase 3 clinical trial in 58 patients aged 6 through 11 years, mean age 9 years (Trial 3). Trial 3 evaluated subjects with a screening ppFEV1 ≥40 [mean ppFEV1 91.4 at baseline (range: 55 to 122.7)]. The safety profile of lumacaftor and ivacaftor in children 6 through 11 years of age was similar to those in patients 12 years and older.
In Trial 3, spirometry (ppFEV1) was assessed as a planned safety endpoint. The within-group LS mean absolute change from baseline in ppFEV1 at Week 24 was 2.5 percentage points. At the Week 26 safety follow-up visit (following a planned discontinuation) ppFEV1 was also assessed. The within-group LS mean absolute change in ppFEV1 from Week 24 at Week 26 was -3.2 percentage points.
The safety and efficacy of lumacaftor and ivacaftor in patients with CF younger than age 6 years have not been established. Cases of non-congenital lens opacities have been reported in pediatric patients treated with lumacaftor and ivacaftor and ivacaftor, a component of lumacaftor and ivacaftor. Although other risk factors were present in some cases (such as corticosteroid use and exposure to radiation), a possible risk attributable to ivacaftor cannot be excluded.
Juvenile Animal Toxicity Data
In a juvenile toxicology study in which ivacaftor was administered to rats from postnatal days 7 to 35, cataracts were observed at all dose levels, ranging from 0.3 to 2 times the MRHD (based on summed AUCs for ivacaftor and its metabolites at oral doses of 10-50 mg/kg/day). This finding has not been observed in older animals.
Geriatic Use
CF is largely a disease of children and young adults. Clinical trials of lumacaftor and ivacaftor did not include sufficient numbers of patients 65 years of age and over to determine whether they respond differently from younger patients.
Gender
There is no FDA guidance on the use of Lumacaftor and ivacaftor with respect to specific gender populations.
Race
There is no FDA guidance on the use of Lumacaftor and ivacaftor with respect to specific racial populations.
Renal Impairment
Lumacaftor and ivacaftor has not been studied in patients with mild, moderate, or severe renal impairment or in patients with end-stage renal disease. No dose adjustment is necessary for patients with mild and moderate renal impairment. Caution is recommended while using lumacaftor and ivacaftor in patients with severe renal impairment (creatinine clearance less than or equal to 30 mL/min) or end-stage renal disease.
Hepatic Impairment
No dose adjustment is necessary for patients with mild hepatic impairment (Child-Pugh Class A). A dose reduction to 2 tablets in the morning and 1 tablet in the evening is recommended for patients with moderate hepatic impairment (Child-Pugh Class B). Studies have not been conducted in patients with severe hepatic impairment (Child-Pugh Class C), but exposure is expected to be higher than in patients with moderate hepatic impairment. Therefore, use with caution at a maximum dose of 1 tablet in the morning and 1 tablet in the evening, or less, in patients with severe hepatic impairment after weighing the risks and benefits of treatment.
Females of Reproductive Potential and Males
Lumacaftor and ivacaftor may decrease hormonal contraceptive exposure, reducing the effectiveness. Hormonal contraceptives, including oral, injectable, transdermal, and implantable, should not be relied upon as an effective method of contraception when co-administered with Lumacaftor and ivacaftor.
Immunocompromised Patients
There is no FDA guidance one the use of Lumacaftor and ivacaftor in patients who are immunocompromised.
Patients with Severe Lung Dysfunction
The Phase 3 trials (Trials 1 and 2) included 29 patients receiving lumacaftor and ivacaftor with ppFEV1 <40 at baseline. The treatment effect in this subgroup was comparable to that observed in patients with ppFEV1 ≥40.
Patients After Organ Transplantation
Lumacaftor and ivacaftor has not been studied in patients with CF who have undergone organ transplantation. Use in transplanted patients is not recommended due to potential drug-drug interactions.
Administration and Monitoring
Administration
- Lumacaftor and ivacaftor should be taken with fat-containing food.
- Examples of appropriate fat-containing foods include eggs, avocados, nuts, butter, peanut butter, cheese pizza, whole-milk dairy products (such as whole milk, cheese, and yogurt), etc.
- If a patient misses a dose and remembers the missed dose within 6 hours, the patient should take the dose with fat-containing food.
- If more than 6 hours elapsed after the usual dosing time, the patient should skip that dose and resume the normal schedule for the following dose.
- A double dose should not be taken to make up for the forgotten dose.
Monitoring
There is limited information regarding Lumacaftor and ivacaftor Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Lumacaftor and ivacaftor and IV administrations.
Overdosage
There have been no reports of overdose with lumacaftor and ivacaftor.
The highest repeated dose was lumacaftor 1000 mg once daily/ivacaftor 450 mg q12h administered to 49 healthy subjects for 7 days in a trial evaluating the effect of lumacaftor and ivacaftor on electrocardiograms (ECGs). Adverse events reported at an increased incidence of ≥5% compared to the lumacaftor 600 mg/ivacaftor 250 mg dosing period and placebo included: headache (29%), transaminase increased (18%), and generalized rash (10%).
No specific antidote is available for overdose with lumacaftor and ivacaftor. Treatment of overdose consists of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient.
Pharmacology


Mechanism of Action
The CFTR protein is a chloride channel present at the surface of epithelial cells in multiple organs. The F508del mutation results in protein misfolding, causing a defect in cellular processing and trafficking that targets the protein for degradation and therefore reduces the quantity of CFTR at the cell surface. The small amount of F508del-CFTR that reaches the cell surface is less stable and has low channel-open probability (defective gating activity) compared to wild-type CFTR protein.
Lumacaftor improves the conformational stability of F508del-CFTR, resulting in increased processing and trafficking of mature protein to the cell surface. Ivacaftor is a CFTR potentiator that facilitates increased chloride transport by potentiating the channel-open probability (or gating) of the CFTR protein at the cell surface. In vitro studies have demonstrated that both lumacaftor and ivacaftor act directly on the CFTR protein in primary human bronchial epithelial cultures and other cell lines harboring the F508del-CFTR mutation to increase the quantity, stability, and function of F508del-CFTR at the cell surface, resulting in increased chloride ion transport. In vitro responses do not necessarily correspond to in vivo pharmacodynamic response or clinical benefit.
Structure
The active ingredients in lumacaftor and ivacaftor tablets are lumacaftor, which has the following chemical name: 3-[6-({[1-(2,2-difluoro-1,3-benzodioxol-5-yl)cyclopropyl]carbonyl}amino)-3-methylpyridin-2-yl]benzoic acid, and ivacaftor, a CFTR potentiator, which has the following chemical name: N-(2,4-di-tert-butyl-5-hydroxyphenyl)-1,4-dihydro-4-oxoquinoline-3-carboxamide. The molecular formula for lumacaftor is C24H18F2N2O5 and for ivacaftor is C24H28N2O3. The molecular weights for lumacaftor and ivacaftor are 452.41 and 392.49, respectively. The structural formulas are:
Lumacaftor


Lumacaftor is a white to off-white powder that is practically insoluble in water (0.02 mg/mL). Ivacaftor is a white to off-white powder that is practically insoluble in water (<0.05 microgram/mL).
Pharmacodynamics
Sweat Chloride Evaluation
Changes in sweat chloride in response to relevant doses of lumacaftor alone or in combination with ivacaftor were evaluated in a double-blind, placebo-controlled, Phase 2 clinical trial in patients with CF 18 years of age and older either homozygous or heterozygous for the F508del mutation. In that trial, 10 patients (homozygous for F508del) completed dosing with lumacaftor alone 400 mg q12h for 28 days followed by the addition of ivacaftor 250 mg q12h for an additional 28 days and 25 patients (homozygous or heterozygous for F508del) completed dosing with placebo. The treatment difference between lumacaftor 400 mg q12h alone and placebo evaluated as mean change in sweat chloride from baseline to Day 28 compared to placebo was -8.2 mmol/L (95% CI -14, -2). The treatment difference between the combination of lumacaftor 400 mg/ivacaftor 250 mg q12h and placebo evaluated as mean change in sweat chloride from baseline to Day 56 compared to placebo was -11 mmol/L (95% CI -18, -4).
Changes in sweat chloride in response to lumacaftor/ivacaftor were also evaluated in a 24-week, open-label Phase 3 clinical trial (Trial 3) in 58 patients with CF, aged 6 through 11 years (homozygous for F508del) who received lumacaftor 200 mg/ivacaftor 250 mg q12h for 24 weeks. Patients treated with lumacaftor/ivacaftor had a reduction in sweat chloride at Day 15 that was sustained through Week 24. The within-group LS mean absolute change from baseline in sweat chloride was -20.4 mmol/L at Day 15 and -24.8 mmol/L at Week 24. In addition, sweat chloride was also assessed after a 2-week washout period to evaluate the off-drug response. The within-group LS mean absolute change in sweat chloride from Week 24 at Week 26 following the 2-week washout period was 21.3 mmol/L.
There was no direct correlation between decrease in sweat chloride levels and improvement in lung function (ppFEV1).
Cardiac Electrophysiology
The effect of multiple doses of lumacaftor 600 mg once daily/ivacaftor 250 mg q12h and lumacaftor 1000 mg once daily/ivacaftor 450 mg q12h on QTc interval was evaluated in a randomized, placebo- and active-controlled (400 mg moxifloxacin), parallel, thorough QT study in 168 healthy subjects. No meaningful changes in QTc interval were observed with either lumacaftor 600 mg once daily/ivacaftor 250 mg q12h and lumacaftor 1000 mg once daily/ivacaftor 450 mg q12h dose groups. A maximum decrease in mean heart rate of up to 8 beats per minute (bpm) from baseline was observed with lumacaftor/ivacaftor treatment. In Trials 1 and 2, a similar decrease in heart rate was observed in patients during initiation of lumacaftor and ivacaftor (lumacaftor 400 mg/ivacaftor 250 mg q12h).
Pharmacokinetics
The exposure (AUC) of lumacaftor is approximately 2-fold higher in healthy adult volunteers compared to exposure in patients with CF. The exposure of ivacaftor is similar between healthy adult volunteers and patients with CF. After twice-daily dosing, steady-state plasma concentrations of lumacaftor and ivacaftor in healthy subjects were generally reached after approximately 7 days of treatment, with an accumulation ratio of approximately 1.9 for lumacaftor. The steady-state exposure of ivacaftor is lower than that of Day 1 due to the CYP3A induction effect of lumacaftor.

Absorption
When a single dose of lumacaftor/ivacaftor was administered with fat-containing foods, lumacaftor exposure was approximately 2 times higher and ivacaftor exposure was approximately 3 times higher than when taken in a fasting state.
Following multiple oral dose administration of lumacaftor in combination with ivacaftor, the exposure of lumacaftor generally increased proportional to dose over the range of 200 mg every 24 hours to 400 mg every 12 hours. The median (range) tmax of lumacaftor is approximately 4.0 hours (2.0; 9.0) in the fed state.
Following multiple oral dose administration of ivacaftor in combination with lumacaftor, the exposure of ivacaftor generally increased with dose from 150 mg every 12 hours to 250 mg every 12 hours. The median (range) tmax of ivacaftor is approximately 4.0 hours (2.0; 6.0) in the fed state.
Distribution
Lumacaftor is approximately 99% bound to plasma proteins, primarily to albumin. After oral administration of 200 mg every 24 hours for 28 days to patients with CF in a fed state, the mean (±SD) for apparent volumes of distribution was 86.0 (69.8) L.
Ivacaftor is approximately 99% bound to plasma proteins, primarily to alpha 1-acid glycoprotein and albumin.
Elimination
The half-life of lumacaftor is approximately 26 hours in patients with CF. The typical apparent clearance, CL/F (CV), of lumacaftor was estimated to be 2.38 L/hr (29.4%) for patients with CF. The half-life of ivacaftor when given with lumacaftor is approximately 9 hours in healthy subjects. The typical CL/F (CV) of ivacaftor when given in combination with lumacaftor was estimated to be 25.1 L/hr (40.5%) for patients with CF.
Lumacaftor is not extensively metabolized in humans with the majority of lumacaftor excreted unchanged in the feces. In vitro and in vivo data indicate that lumacaftor is mainly metabolized via oxidation and glucuronidation.
Ivacaftor is extensively metabolized in humans. In vitro and in vivo data indicate that ivacaftor is primarily metabolized by CYP3A. M1 and M6 are the two major metabolites of ivacaftor in humans.
Excretion
Following oral administration of lumacaftor, the majority of lumacaftor (51%) is excreted unchanged in the feces. There was minimal elimination of lumacaftor and its metabolites in urine (only 8.6% of total radioactivity was recovered in the urine with 0.18% as unchanged parent).
Following oral administration of ivacaftor alone, the majority of ivacaftor (87.8%) is eliminated in the feces after metabolic conversion. There was minimal elimination of ivacaftor and its metabolites in urine (only 6.6% of total radioactivity was recovered in the urine).
Specific Populations
Age: Pediatric Population
The following conclusions about exposures between adults and the pediatric population are based on population pharmacokinetics (PK) analyses:
Pediatric patients 6 through 11 years of age
Following oral administration of lumacaftor and ivacaftor tablets, lumacaftor 200 mg/ivacaftor 250 mg every 12 hours, the mean lumacaftor (±SD) AUCss was 203 (57.4) µg/mL*h and is comparable to the mean AUCss in patients 12 years and older administered Lumacaftor and ivacaftor tablets, lumacaftor 400 mg/ivacaftor 250 mg every 12 hours. The mean ivacaftor (±SD) AUCss was 5.26 (3.08) µg/mL*h and is comparable to the mean AUCs in patients 12 years and older administered lumacaftor and ivacaftor tablets, lumacaftor 400 mg/ivacaftor 250 mg every 12 hours.
Pediatric patients 12 to less than 18 years of age
Following oral administration of lumacaftor and ivacaftor tablets, lumacaftor 400 mg/ivacaftor 250 mg every 12 hours, the mean lumacaftor (±SD) AUCss was 241 (61.4) µg/mL*h and is comparable to the mean AUCss in adult patients administered lumacaftor and ivacaftor tablets, lumacaftor 400 mg/ivacaftor 250 mg every 12 hours. The mean [[[ivacaftor]] (±SD) AUCss was 3.90 (1.56) µg/mL*h and is comparable to the mean AUCss in adult patients administered lumacaftor and ivacaftor tablets, lumacaftor 400 mg/ivacaftor 250 mg every 12 hours.
Sex
The pharmacokinetics of lumacaftor and ivacaftor was evaluated using a population PK analysis of data from clinical studies of lumacaftor given in combination with ivacaftor. Results indicate no clinically relevant difference in pharmacokinetic parameters for lumacaftor and ivacaftor between males and females.
Pharmacokinetic studies have not been performed with lumacaftor and ivacaftor in patients with renal impairment.
Following multiple doses of lumacaftor/ivacaftor for 10 days, subjects with moderately impaired hepatic function (Child-Pugh Class B, score 7 to 9) had approximately 50% higher exposures (AUC0-12h) and approximately 30% higher Cmax for both lumacaftor and ivacaftor compared with healthy subjects matched for demographics. Pharmacokinetic studies have not been conducted in patients with mild (Child-Pugh Class A, score 5 to 6) or severe hepatic impairment (Child-Pugh Class C, score 10 to 15) receiving lumacaftor and ivacaftor.
Drug Interaction Studies
Drug interaction studies were performed with lumacaftor/ivacaftor and other drugs likely to be co-administered or drugs commonly used as probes for pharmacokinetic interaction studies.
Potential for Lumacaftor/Ivacaftor to Affect Other Drugs
Lumacaftor is a strong inducer of CYP3A. Co-administration of lumacaftor with ivacaftor, a sensitive CYP3A substrate, decreased ivacaftor exposure by 80%. Ivacaftor is a weak inhibitor of CYP3A when given as monotherapy. The net effect of lumacaftor/ivacaftor therapy is strong CYP3A induction.
Based on in vitro results which showed P-gp inhibition and PXR activation, lumacaftor has the potential to both inhibit and induce P-gp. A clinical study with ivacaftor monotherapy showed that ivacaftor is a weak inhibitor of P-gp. Therefore, concomitant use of lumacaftor and ivacaftor with P-gp substrates may alter the exposure of these substrates.
In vitro studies suggest that lumacaftor has the potential to induce CYP2B6, CYP2C8, CYP2C9, and CYP2C19; inhibition of CYP2C8 and CYP2C9 has also been observed in vitro. In vitro studies suggest that ivacaftor may inhibit CYP2C9. Therefore, concomitant use of lumacaftor and ivacaftor with CYP2B6, CYP2C8, CYP2C9, and CYP2C19 substrates may alter the exposure of these substrates.
Potential for Other Drugs to Affect Lumacaftor/Ivacaftor
Lumacaftor exposure is not affected by concomitant CYP3A inducers or inhibitors. Exposure of ivacaftor when given in combination with lumacaftor is reduced by concomitant CYP3A inducers and increased by concomitant CYP3A inhibitors.
The effects of co-administered drugs on the exposure of lumacaftor and ivacaftor are shown in Table 4.

Nonclinical Toxicology
Carcinogenesis,Mutagenesis, Impairment of Fertility
No studies of carcinogenicity, mutagenicity, or impairment of fertility were conducted with lumacaftor and ivacaftor; however, studies are available for individual components, lumacaftor and ivacaftor, as described below.
Lumacaftor
A two-year study in Sprague-Dawley rats and a 26-week study in transgenic Tg.rasH2 mice were conducted to assess carcinogenic potential of lumacaftor. No evidence of tumorigenicity was observed in rats at lumacaftor oral doses up to 1000 mg/kg/day (approximately 5 and 13 times the MRHD on a lumacaftor AUC basis in males and females, respectively). No evidence of tumorigenicity was observed in Tg.rasH2 mice at lumacaftor oral doses up to 1500 and 2000 mg/kg/day in female and male mice, respectively. Lumacaftor was negative for genotoxicity in the following assays: Ames test for bacterial gene mutation, in vitro chromosomal aberration assay in Chinese hamster ovary cells, and in vivo mouse micronucleus test.
Lumacaftor had no effects on fertility and reproductive performance indices in male and female rats at an oral dose of 1000 mg/kg/day (approximately 3 and 8 times, respectively, the MRHD on a lumacaftor AUC basis).
Two-year studies were conducted in mice and rats to assess carcinogenic potential of ivacaftor. No evidence of tumorigenicity was observed in mice and rats at ivacaftor oral doses up to 200 mg/kg/day and 50 mg/kg/day, respectively (approximately equivalent to 3 and 10 times the MRHD based on summed AUCs of ivacaftor and its metabolites).
Ivacaftor was negative for genotoxicity in the following assays: Ames test for bacterial gene mutation, in vitro chromosomal aberration assay in Chinese hamster ovary cells, and in vivo mouse micronucleus test.
Ivacaftor impaired fertility and reproductive performance indices in male and female rats at an oral dose of 200 mg/kg/day (approximately 15 and 7 times the MRHD based on summed AUCs of ivacaftor and its metabolites). Increases in prolonged diestrus were observed in females at 200 mg/kg/day. Ivacaftor also increased the number of females with all nonviable embryos and decreased corpora lutea, implantations, and viable embryos in rats at 200 mg/kg/day (approximately 7 times the MRHD based on summed AUCs of ivacaftor and its metabolites) when dams were dosed prior to and during early pregnancy. These impairments of fertility and reproductive performance in male and female rats at 200 mg/kg/day were attributed to severe toxicity. No effects on male or female fertility and reproductive performance indices were observed at an oral dose of ≤100 mg/kg/day (approximately 8 and 5 times the MRHD based on summed AUCs of ivacaftor and its metabolites).
Clinical Studies
Dose Ranging
Dose ranging for the clinical program consisted primarily of one double-blind, placebo-controlled, multiple-cohort trial which included 97 Caucasian patients with CF (homozygous for the F508del mutation) 18 years of age and older with a screening ppFEV1 ≥40. In the trial, 76 patients (homozygous for the F508del mutation) were randomized to receive lumacaftor alone at once-daily doses of 200 mg, 400 mg, or 600 mg or 400 mg q12h for 28 days followed by the addition of ivacaftor 250 mg q12h and 27 patients (homozygous or heterozygous for the F508del mutation) received placebo. During the initial 28-day lumacaftor monotherapy period, treatment with lumacaftor demonstrated a dose-dependent decrease in ppFEV1 compared to placebo. Changes from Day 1 at Day 28 in ppFEV1 compared to placebo were 0.24, -1.4, -2.7, and -4.6 for the 200 mg once daily, 400 mg once daily, 600 mg once daily, and 400 mg q12h lumacaftor doses, respectively. Following the addition of ivacaftor 250 mg q12h, the changes from Day 1 at Day 56 in ppFEV1 compared to placebo were 3.8, 2.7, 5.6, and 4.2, respectively.
Sweat chloride was also assessed in this trial. Following the initial 28 days of lumacaftor monotherapy, the changes from Day 1 at Day 28 in sweat chloride compared to placebo were -4.9, -8.3, -6.1, and -8.2 mmol/L for the 200 mg once daily, 400 mg once daily, 600 mg once daily, and 400 mg q12h lumacaftor doses, respectively. Following the addition of ivacaftor 250 mg q12h, the changes from Day 1 at Day 56 in sweat chloride compared to placebo were -5.0, -9.8, -9.5, and -11 mmol/L, respectively.
These data supported the evaluation of lumacaftor 400 mg/ivacaftor 250 mg q12h and lumacaftor 600 mg once daily/ivacaftor 250 mg q12h in the confirmatory trials.
Confirmatory
The efficacy of lumacaftor and ivacaftor in patients with CF who are homozygous for the F508del mutation in the CFTR gene was evaluated in two randomized, double-blind, placebo-controlled, 24-week clinical trials (Trials 1 and 2) in 1108 clinically stable patients with CF of whom 369 patients received lumacaftor and ivacaftor twice daily.
Trial 1 evaluated 549 patients with CF who were aged 12 years and older (mean age 25.1 years) with ppFEV1 at screening between 40-90 [mean ppFEV1 60.7 at baseline (range: 31.1 to 94.0)]. Trial 2 evaluated 559 patients aged 12 years and older (mean age 25.0 years) with ppFEV1 at screening between 40-90 [mean ppFEV1 60.5 at baseline (range: 31.3 to 99.8)]. Patients with a history of colonization with organisms such as Burkholderia cenocepacia, Burkholderia dolosa, or Mycobacterium abscessus, or who had 3 or more abnormal liver function tests (ALT, AST, AP, GGT ≥3 × the ULN or total bilirubin ≥2 × the ULN) were excluded.
Patients in both trials were randomized 1:1:1 to receive either lumacaftor and ivacaftor (lumacaftor 400 mg q12h/ivacaftor 250 mg q12h; or lumacaftor 600 mg once daily/ivacaftor 250 mg q12h) or placebo. Patients took the study drug with fat-containing food for 24 weeks in addition to their prescribed CF therapies (e.g., bronchodilators, inhaled antibiotics, dornase alfa, and hypertonic saline).
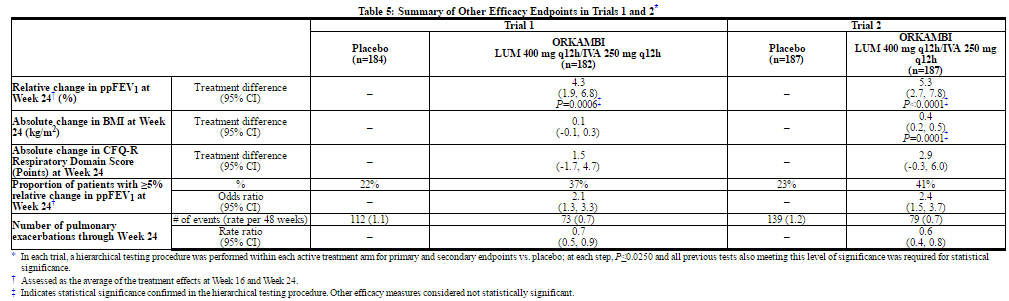
The primary efficacy endpoint in both trials was change in lung function as determined by absolute change from baseline in ppFEV1 at Week 24, assessed as the average of the treatment effects at Week 16 and at Week 24. In both trials, treatment with lumacaftor and ivacaftor resulted in a statistically significant improvement in ppFEV1. The treatment difference between lumacaftor and ivacaftor and placebo for the mean absolute change in ppFEV1 from baseline at Week 24 (assessed as the average of the treatment effects at Week 16 and at Week 24) was 2.6 percentage points [95% CI (1.2, 4.0)] in Trial 1 (P=0.0003) and 3.0 percentage points [95% CI (1.6, 4.4)] in Trial 2 (P<0.0001). These changes persisted throughout the 24-week treatment period (Figure 1). Improvements in ppFEV1 were observed regardless of age, disease severity, sex, and geographic region.

Key secondary efficacy variables included relative change from baseline in ppFEV1 at Week 24, assessed as the average of the treatment effects at Week 16 and at Week 24; absolute change from baseline in BMI at Week 24; absolute change from baseline in Cystic Fibrosis Questionnaire-Revised (CFQ-R) Respiratory Domain score at Week 24, a measure of respiratory symptoms relevant to patients with CF such as cough, sputum production, and difficulty breathing; proportion of patients achieving ≥5% relative change from baseline in ppFEV1 using the average of Week 16 and Week 24; and number of pulmonary exacerbations through Week 24. For the purposes of these trials, a pulmonary exacerbation was defined as a change in antibiotic therapy (IV, inhaled, or oral) as a result of 4 or more of 12 pre-specified sino-pulmonary signs/symptoms.
How Supplied
LUMACAFTOR AND IVACAFTOR (lumacaftor 200 mg/ivacaftor 125 mg) is supplied as pink, oval-shaped tablets; each tablet contains 200 mg of lumacaftor and 125 mg of ivacaftor, printed with "2V125" in black ink on one side and plain on the other, and is packaged as follows:

LUMACAFTOR AND IVACAFTOR (lumacaftor 100 mg/ivacaftor 125 mg) is supplied as pink, oval-shaped tablets; each tablet contains 100 mg of lumacaftor and 125 mg of ivacaftor, printed with "1V125" in black ink on one side and plain on the other, and is packaged as follows:

Storage
Store at 20-25°C (68-77°F); excursions permitted to 15-30°C (59-86°F).
Images
Drug Images
{{#ask: Page Name::Lumacaftor and ivacaftor |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel




{{#ask: Label Page::Lumacaftor and ivacaftor |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
- Advanced Liver Disease
- Inform patients that worsening of liver function in patients with advanced liver disease occurred in some patients treated with lumacaftor and ivacaftor.
- If lumacaftor and ivacaftor is used in these patients, they should be closely monitored after the initiation of treatment and the dose should be reduced.
- Abnormalities in Liver Function and Testing
- Inform patients that abnormalities in liver function have occurred in patients treated with lumacaftor and ivacaftor.
- Blood tests to measure transaminases (ALT and AST) and bilirubin will be performed prior to initiating lumacaftor and ivacaftor, every 3 months during the first year of therapy, and annually thereafter.
- Respiratory Events
- Inform patients that chest discomfort, dyspnea, and respiration abnormal were more common during initiation of lumacaftor and ivacaftor therapy.
- Additional monitoring of patients with ppFEV1 <40 is recommended during initiation of therapy.
- Effect on Blood Pressure
- Inform patients that increased blood pressure has been observed in some patients treated with lumacaftor and ivacaftor and that periodic monitoring of their blood pressure during treatment is recommended.
- Drug Interactions with CYP3A Inhibitors and Inducer
- Ask patients to tell you all the medications they are taking, including any herbal supplements or vitamins.
- Co–administration with sensitive CYP3A substrates or CYP3A substrates with a narrow therapeutic index is not recommended.
- Instruct patients on alternative methods of birth control because hormonal contraceptives should not be relied upon as an effective method of contraception and there is an increased incidence of menstruation-related adverse reactions when co-administered with lumacaftor and ivacaftor.
- When initiating lumacaftor and ivacaftor in patients taking strong CYP3A inhibitors (e.g., itraconazole), instruct the patient to reduce the dose of lumacaftor and ivacaftor to 1 tablet daily for the first week of treatment. Following this period, continue with the recommended daily dose.
- Patients should be instructed to tell their doctor if they stop lumacaftor and ivacaftor for more than 1 week while they are also taking a strong CYP3A inhibitor because the dose of lumacaftor and ivacaftor would need to be reduced upon re-initiation.
- The dose of lumacaftor and ivacaftor should be reduced to 1 tablet daily for the first week upon treatment re-initiation. Following this period, continue with the recommended daily dose.
- Use in Patients with Hepatic Impairment
- Inform patients with moderate hepatic impairment (Child-Pugh Class B) to reduce the dose of lumacaftor and ivacaftor to 2 tablets in the morning and 1 tablet in the evening.
- If initiating lumacaftor and ivacaftor in a patient with severe hepatic impairment, after weighing the risks and benefits of treatment, instruct the patient to take a maximum dose of 1 tablet every 12 hours, or less.
- Administration
- Inform patients that lumacaftor and ivacaftor is best absorbed by the body when taken with fat-containing food.
- A typical CF diet will satisfy this requirement. Examples of fat-containing foods include eggs, avocados, nuts, butter, peanut butter, cheese pizza, whole-milk dairy products (such as whole milk, cheese, and yogurt), etc.
- Inform patients that if a dose is missed and they remember the missed dose within 6 hours, the patients should take the dose with fat-containing food.
- If more than 6 hours elapsed after the usual dosing time, the patients should skip that dose and resume the normal schedule for the following dose.
- Patients should be informed not to take a double dose to make up for the forgotten dose.
- Cataracts
Precautions with Alcohol
Alcohol-Lumacaftor and ivacaftor interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
ORKAMBI
Look-Alike Drug Names
There is limited information regarding Lumacaftor and ivacaftor Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.