Glycerophospholipid
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
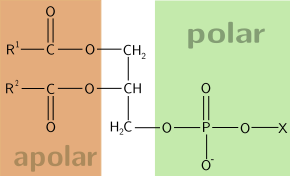
Glycerophospholipids or phosphoglycerides are glycerol-based phospholipids. They are the main component of biological membranes.
Structures
The term glycerophospholipid signifies any derivative of sn-glycero-3-phosphoric acid that contains at least one O-acyl, or O-alkyl or O-alk-1'-enyl residue attached to the glycerol moiety and a polar head made of a nitrogenous base, a glycerol, or an inositol unit.
It contains a glycerol core with fatty acids. They can be the same or different subunits of fatty acids.
- Carbon 1 (tail, apolar) contains a fatty acid, typically saturated
- Carbon 2 (tail, apolar) contains a fatty acid, typically unsaturated and in the cis conformation, thus appearing "bent"
- Carbon 3 (head, polar) contains a phosphate group or an alcohol attached to a phosphate group
Nomenclature and stereochemistry
Glycerophospholipids generally use a "sn" notation which stands for stereochemical numbering. When the letters "sn" appear in the nomenclature, by convention the hydroxyl group of the second carbon of glycerol (sn-2) is on the left on a Fischer projection. The numbering follows the one of Fischer's projections, being sn-1 the carbon at the top and sn-3 the one at the bottom.
The advantage of this particular notation is that the spatial conformation (R or L) of the glycero-molecule is determined intuitively by the residues on the positions sn-1 and sn-3.
For example sn-glycero-3-phosphoric acid and sn-glycero-1-phosphoric acid are enantiomers.
Examples of glycerophospholipids
| Name | Image | Head | Image | Charge |
| Phosphatidyl choline (lecithin) |  |
choline | 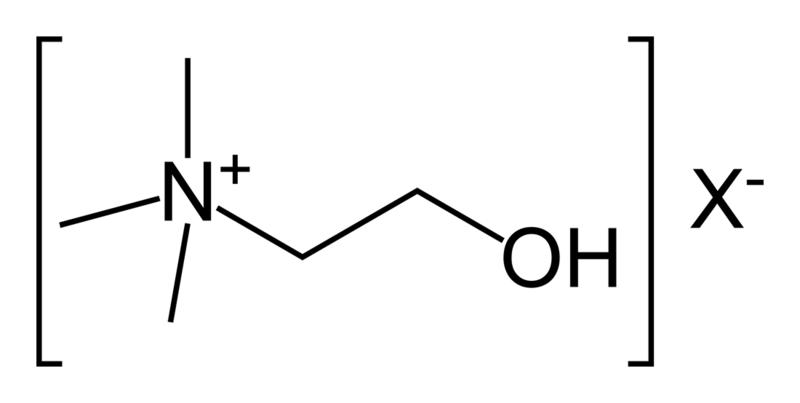 |
neutral |
| Phosphatidyl ethanolamine (cephalin) | File:Phosphatidyl-Ethanolamine.png | ethanolamine | File:Ethanolamine.png | neutral |
| Phosphatidyl inositol | File:Phosphatidyl-Inositol.png | inositol | File:Inositol chair.png | negative |
| Phosphatidyl serine | 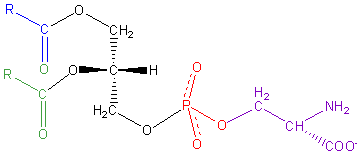 |
serine | File:L-serine-skeletal.png | negative |
| Bisphosphatidyl glycerol (cardiolipin) | File:Diphosphatidyl-Glycerol.png | - | - | negative |
Lecithin and cephalin are more common than the others in most human membranes, but cardiolipin is quite common in the inner membranes of mitochondria.
Uses
Use in membranes
One of a glycerophospholipid's functions is to serve as a structural component of cell membranes. The cell membrane seen under the electron microscope consists of two identifiable layers, or "leaflets", each of which is made up of an ordered row of glycerophospholipid molecules. The composition of each layer can vary widely depending on the type of cell.
- For example, in human erythrocytes the cytosolic side (the side facing the cytosol) of the plasma membrane consists mainly of phosphatidylethanolamine, phosphatidylserine, and phosphatidylinositol.
- By contrast, the exoplasmic side (the side on the exterior of the cell) consists mainly of phosphatidylcholine and sphingomyelin, a type of sphingolipid.
Each glycerophospholipid molecule consists of a small polar head group and two long hydrophobic chains. In the cell membrane, the two layers of phospholipids are arranged as as follows:
- the hydrophobic tails point to each other and form a fatty, hydrophobic center
- the ionic head groups are placed at the inner and outer surfaces of the cell membrane
This is a stable structure because the ionic hydrophilic head groups interact with the aqueous media inside and outside the cell, whereas the hydrophobic tails maximize hydrophobic interactions with each other and are kept away from the aqueous environments. The overall result of this structure is to construct a fatty barrier between the cell's interior and its surroundings.
Use in emulsification
Glycerophospholipids can also act as an emulsifying agent to promote dispersal of one substance into another. This is sometimes used in candy making.
External links
See also
External links
- Glycerophospholipids at the US National Library of Medicine Medical Subject Headings (MeSH)