Glucose-6-phosphate dehydrogenase deficiency pathophysiology
|
Glucose-6-phosphate dehydrogenase deficiency Microchapters |
|
Differentiating Glucose-6-phosphate dehydrogenase deficiency from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Glucose-6-phosphate dehydrogenase deficiency pathophysiology On the Web |
|
American Roentgen Ray Society Images of Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
|
FDA on Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
|
CDC on Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
|
Glucose-6-phosphate dehydrogenase deficiency pathophysiology in the news |
|
Blogs on Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
|
Directions to Hospitals Treating Glucose-6-phosphate dehydrogenase deficiency |
|
Risk calculators and risk factors for Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
- Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mahda Alihashemi M.D. [2]
Overview
It is understood that G6PD deficiency is the result of reduced Glucose-6-phosphate dehydrogenase enzyme levels. G6PD deficiency is an X-linked disorder. Glucose-6-phosphate dehydrogenase enzyme oxidizes glucose-6-phosphate to 6-phosphogluconolactone in pentose phosphate pathway ( HMP shunt). Glucose-6-phosphate dehydrogenase enzyme also reduces nicotinamide adenine dinucleotide phosphate (NADP) to NADPH. NADPH is an important cofactor in glutathione metabolism against oxidative injury in RBC. In G6PD deficiency, oxidative stresses can denature hemoglobin and intravascular hemolysis in RBC can happen. The gene G6PD is located in the distal long arm of the X chromosome at the Xq28 locus. G6PD B, is the wild type or normal. On microscopic histopathological analysis, Heinz bodies can be visualized as a result of denatured hemoglobin in peripheral blood smears with supravital staining.
Pathophysiology
Physiology
The normal physiology of G6PD deficiency can be understood as follows:
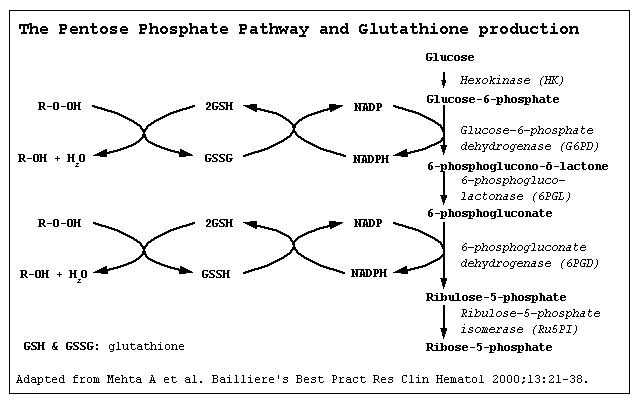
Pathogenesis
- It is understood that G6PD deficiency is the result of reduced Glucose-6-phosphate dehydrogenase enzyme levels. G6PD deficiency is an X-linked disorder. It is the most common enzymatic disorder of red blood cells. Glucose-6-phosphate dehydrogenase enzyme oxidizes glucose-6-phosphate to 6-phosphogluconolactone in pentose phosphate pathway ( HMP shunt). Glucose-6-phosphate dehydrogenase enzyme also reduces nicotinamide adenine dinucleotide phosphate (NADP) to NADPH. NADPH is an important cofactor in glutathione metabolism against oxidative injury in RBC. Reduced glutathione (GSH) convert to oxidized glutathione (GSSG) by glutathione peroxidase enzyme that prevents oxidant accumulation. Glutathione reductase catalyzes the reduction of GSSG to GSH by NADPH. In G6PD deficiency, oxidative stresses can denature hemoglobin and intravascular hemolysis in RBC can happen. Infection, some medications and foods with high level of convicine, vicine, divicine and isouramil such as fava beans can cause oxidative stress. The spleen is the organ for sequestration damaged RBC. The hemoglobin is metabolized to bilirubin and cause jaundice.
Genetics
G6PD deficiency is transmitted in x-linked disorder pattern. The gene G6PD is located in the distal long arm of the X chromosome at the Xq28 locus. [1]
Heterozygous women are usually normal because of lyonization ( X innactivation)[2]
G6PD B, is the wild type or normal. G6PD has 400 variant enzymes. [3] Caucasians, Asians and majority of blacks has G6PD B.
G6PD A+: In Africa, in 20-30 percent of black. In this variant, asparagine is substitued for aspartate, at amino acid 126. [4] It has normal enzyme activity.
The development of G6PD deficency is the result of missense point mutations and also a few deletions. [5]
- G6PD A+:In Africa, in 20-30 percent of black. In this variant, asparagine is substitued for aspartate, at amino acid 126. [4] It has normal enzyme activity.
- G6PD A-: Cause primaquine sensitivity in blacks.
- G6PD mediterranean variant: Single base substitution (C—>T) at nucleotide 563 [6]
- G6PD variants in Asia:
- In China: G6PD Canton (1376 G—>T), G6PD Kaiping (1388 G—>A), G6PD Gaohe (95 G—>A)[7]
- In Southeast Asia: G6PD Mahidol (487G—>A)
Associated Conditions
- G6PD A− and G6PD Mediterranean has protective effect against Plasmodium falciparum and Plasmodium vivax malaria. [8]
- G6PD deficency is a risk factor of male neonatal sepsis[9]
Gross Pathology
On gross pathology,there are no characteristic findings of G6PD deficiency.
Microscopic Pathology
On microscopic histopathological analysis, Heinz bodies can be visualized as a result of denatured hemoglobin in peripheral blood smears with supravital staining. (Heinz body prep).[10]
 |
References
- ↑ KIRKMAN HN, HENDRICKSON EM (September 1963). "Sex-linked electrophoretic difference in glucose-6-phosphate dehydrogenase". Am. J. Hum. Genet. 15: 241–58. PMC 1932381. PMID 14033020.
- ↑ BEUTLER E, YEH M, FAIRBANKS VF (January 1962). "The normal human female as a mosaic of X-chromosome activity: studies using the gene for C-6-PD-deficiency as a marker". Proc. Natl. Acad. Sci. U.S.A. 48: 9–16. PMC 285481. PMID 13868717.
- ↑ Beutler E (December 1994). "G6PD deficiency". Blood. 84 (11): 3613–36. PMID 7949118.
- ↑ 4.0 4.1 Yoshida A (March 1967). "A single amino Acid substitution (asparagine to aspartic Acid) between normal (b+) and the common negro variant (a+) of human glucose-6-phosphate dehydrogenase". Proc. Natl. Acad. Sci. U.S.A. 57 (3): 835–40. PMC 335583. PMID 16591538.
- ↑ Beutler E (April 1990). "The genetics of glucose-6-phosphate dehydrogenase deficiency". Semin. Hematol. 27 (2): 137–64. PMID 2190319.
- ↑ Vulliamy TJ, D'Urso M, Battistuzzi G, Estrada M, Foulkes NS, Martini G, Calabro V, Poggi V, Giordano R, Town M (July 1988). "Diverse point mutations in the human glucose-6-phosphate dehydrogenase gene cause enzyme deficiency and mild or severe hemolytic anemia". Proc. Natl. Acad. Sci. U.S.A. 85 (14): 5171–5. PMC 281710. PMID 3393536.
- ↑ McCurdy PR, Kirkman HN, Naiman JL, Jim RT, Pickard BM (March 1966). "A Chinese variant of glucose-6-phosphate dehydrogenase". J. Lab. Clin. Med. 67 (3): 374–85. PMID 4379606.
- ↑ Nagel RL, Roth EF (September 1989). "Malaria and red cell genetic defects". Blood. 74 (4): 1213–21. PMID 2669996.
- ↑ Rostami-Far Z, Ghadiri K, Rostami-Far M, Shaveisi-Zadeh F, Amiri A, Rahimian Zarif B (2016). "Glucose-6-phosphate dehydrogenase deficiency (G6PD) as a risk factor of male neonatal sepsis". J Med Life. 9 (1): 34–38. PMC 5152609. PMID 27974910.
- ↑ Jacob HS (July 1970). "Mechanisms of Heinz body formation and attachment to red cell membrane". Semin. Hematol. 7 (3): 341–54. PMID 5425759.
- ↑ From en.wikipedia.org, Public Domain, <"https://commons.wikimedia.org/w/index.php?curid=">4647353