Flosequinan
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
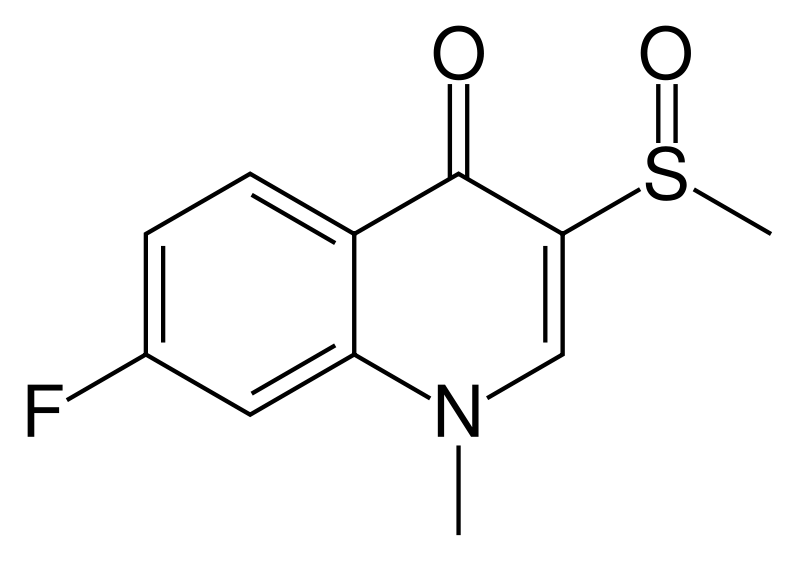
| Formula | C11H10FNO2S |
| Molar mass | 239.267 g/mol |
|
WikiDoc Resources for Flosequinan |
|
Articles |
|---|
|
Most recent articles on Flosequinan Most cited articles on Flosequinan |
|
Media |
|
Powerpoint slides on Flosequinan |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Flosequinan at Clinical Trials.gov Clinical Trials on Flosequinan at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Flosequinan
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Flosequinan Discussion groups on Flosequinan Patient Handouts on Flosequinan Directions to Hospitals Treating Flosequinan Risk calculators and risk factors for Flosequinan
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Flosequinan |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Flosequinan is a quinolone vasodilator. It has direct relaxing effects on peripheral arteries and veins. It is administered orally in cases of congestive heart failure in patients who are not responsive to digitalis or ACE inhibitors. It was sold under the trade name Manoplax.
It was withdrawn from the US market in October 1993 due to an increased risk of hospitalization or death.[1]
Synthesis
Both methods for forming the heterocyclic ring in quinolones involved cyclization into the carbocyclic ring. A closely related quinolone that displays cardiovascular rather than antibiotic activity is constructed by a condensation that closes the bond at the 2,3 position in the heterocyclic ring.
![700px U.S. Patent 5,079,264 N.B.[2]](/images/f/f7/Flosequinan_Syn.png)
The starting material (2) is obtained by reaction of the aminoacetophenone derivative (1) with ethyl formate. Heating the product in ethylene glycol methyl ether leads to an aldol-like cyclization and the formation of a quinolone ring. The product, flosequinan (3), displays vasodilator and cardiotonic activities.
See also
References
- ↑ "Heart drug withdrawn - Boots Pharmaceuticals' Manoplax". FDA Consumer. 1993. Retrieved 2008-12-01.
- ↑ http://www.druglead.com/cds/flosequinan.html
- Pages with script errors
- Pages using duplicate arguments in template calls
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Withdrawn drugs
- Vasodilators
- Quinolines
- Sulfoxides
- Ketones
- Organofluorides