Isosorbide mononitrate
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Isosorbide mononitrate is an anti-anginal nitrate that is FDA approved for the {{{indicationType}}} of angina pectoris due to coronary artery disease.. Common adverse reactions include dizziness and headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Prophylaxis of Angina Pectoris
- Dosing Information
- The recommended starting dose is 30 mg (given as a single 30 mg tablet or as 1/2 of a 60 mg tablet) or 60 mg (given as a single tablet) once daily.
- After several days, the dosage may be increased to 120 mg (given as a single 120 mg tablet or as two 60 mg tablets) once daily. Rarely, 240 mg may be required.
- The daily dose of Imdur tablets should be taken in the morning on arising.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Isosorbide mononitrate in adult patients.
Non–Guideline-Supported Use
Prophylaxis of Rebleeding of Esophageal Varices
- Dosing Information
- 10 mg twice daily and titrated to 20 mg twice daily unless hypotension or headache occurred.[1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Isosorbide mononitrate in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Isosorbide mononitrate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Isosorbide mononitrate in pediatric patients.
Contraindications
- Hypersensitivity or synsyncratic reactions to other nitrates or nitrites.
Warnings
- Amplification of the vasodilatory effects of Imdur by sildenafil can result in severe hypotension. The time course and dose dependence of this interaction have not been studied. Appropriate supportive care has not been studied, but it seems reasonable to treat this as a nitrate overdose, with elevation of the extremities and with central volume expansion.
- The benefits of ISMN in patients with acute myocardial infarction or congestive heart failure have not been established; because the effects of isosorbide mononitrate are difficult to terminate rapidly, this drug is not recommended in these settings.
- If isosorbide mononitrate is used in these conditions, careful clinical or hemodynamic monitoring must be used to avoid the hazards of hypotension and tachycardia.
Precautions
- Severe hypotension, particularly with upright posture, may occur with even small doses of isosorbide mononitrate. This drug should, therefore, be used with caution in patients who may be volume depleted or who, for whatever reason, are already hypotensive. Hypotension induced by isosorbide mononitrate may be accompanied by paradoxical bradycardia and increased angina pectoris.
- Nitrate therapy may aggravate the angina caused by hypertrophic cardiomyopathy.
- In industrial workers who have had long-term exposure to unknown (presumably high) doses of organic nitrates, tolerance clearly occurs. Chest pain, acute myocardial infarction, and even sudden death have occurred during temporary withdrawal of nitrates from these workers, demonstrating the existence of true physical dependence. The importance of these observations to the routine, clinical use of oral isosorbide mononitrate is not known.
Adverse Reactions
Clinical Trials Experience
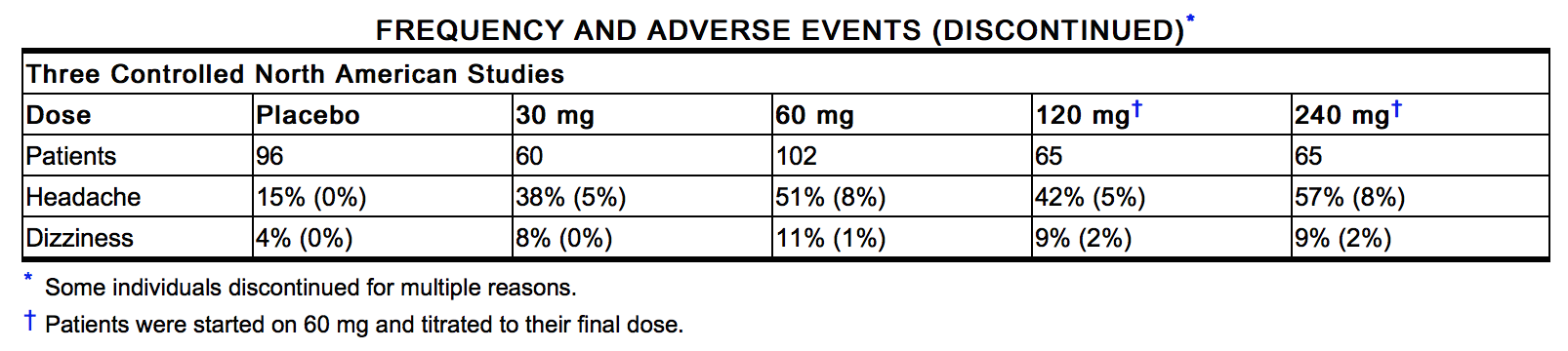
- The table below shows the frequencies of the adverse events that occurred in >5% of the subjects in three placebo-controlled North American studies in which patients in the active treatment arm received 30 mg, 60 mg, 120 mg, or 240 mg of isosorbide mononitrate as Imdur tablets once daily. In parentheses, the same table shows the frequencies with which these adverse events were associated with the discontinuation of treatment. Overall, 8% of the patients who received 30 mg, 60 mg, 120 mg, or 240 mg of isosorbide mononitrate in the three placebo-controlled North American studies discontinued treatment because of adverse events. Most of these discontinued because of headache. Dizziness was rarely associated with withdrawal from these studies. Since headache appears to be a dose-related adverse effect and tends to disappear with continued treatment, it is recommended that Imdur treatment be initiated at low doses for several days before being increased to desired levels.
- In addition, the three North American trials were pooled with 11 controlled trials conducted in Europe. Among the 14 controlled trials, a total of 711 patients were randomized to Imdur tablets. When the pooled data were reviewed, headache and dizziness were the only adverse events that were reported by >5% of patients. Other adverse events, each reported by ≤5% of exposed patients, and in many cases of uncertain relation to drug treatment, were:
Body as a Whole
Asthenia, back pain, chest pain, edema, fatigue, fever, flu-like symptoms, malaise, rigors.
Cardiovascular
Cardiac failure, hypertension, hypotension, arrhythmia, atrial fibrillation, bradycardia, bundle branch block, extrasystole, palpitation, tachycardia, ventricular tachycardia, angina pectoris aggravated, heart murmur, myocardial infarction, Q wave abnormality.
Digestive
Abdominal pain, constipation, diarrhea, dyspepsia, flatulence, gastric ulcer, gastritis, glossitis, hemorrhagic gastric ulcer, hemorrhoids, loose stools, melena, nausea, vomiting, SGOT increase, SGPT increase.
Endocrine
Hematologic and Lymphatic
Purpura, thrombocytopenia, hypochromic anemia.
Vascular
Flushing, intermittent claudication, leg ulcer, varicose vein.
Musculoskeletal
Arthralgia, frozen shoulder, muscle weakness, musculoskeletal pain, myalgia, myositis, tendon disorder, torticollis.
Neurologic (Autonomic Nervous System)
Dry mouth, hot flushes.
Neurologic
Dizziness, headache, hypoesthesia, migraine, neuritis, paresis, paresthesia, ptosis, tremor, vertigo.
Respiratory
Bronchitis, bronchospasm, cough, dyspnea, increased sputum, nasal congestion, pharyngitis, pneumonia, pulmonary infiltration, rales, rhinitis, sinusitis.
Skin and Hypersensitivy Reactions
Acne, hair texture abnormal, increased sweating, pruritus, rash, skin nodule.
Special Senses
Earache, tinnitus, tympanic membrane perforation, conjunctivitis, photophobia, vision abnormal.
Urogenital
Polyuria, renal calculus, urinary tract infection.
Miscellaneous
Anxiety, concentration impaired, confusion, decreased libido, depression, impotence, insomnia, nervousness, paroniria, somnolence, atrophic vaginitis, breast pain, bacterial infection, moniliasis, viral infection.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Isosorbide mononitrate in the drug label.
Drug Interactions
- The vasodilating effects of isosorbide mononitrate may be additive with those of other vasodilators. Alcohol, in particular, has been found to exhibit additive effects of this variety.
- Marked symptomatic orthostatic hypotension has been reported when calcium channel blockers and organic nitrates were used in combination. Dose adjustments of either class of agents may be necessary.
Drug/Laboratory Test Interactions
- Nitrates and nitrites may interfere with the Zlatkis-Zak color reaction, causing falsely low readings in serum cholesterol determinations.
Use in Specific Populations
Pregnancy
- Pregnancy Category B
- In studies designed to detect effects of isosorbide mononitrate on embryo-fetal development, doses of up to 240 or 248 mg/kg/day, administered to pregnant rats and rabbits, were unassociated with evidence of such effects. These animal doses are about 100 times the maximum recommended human dose (120 mg in a 50 kg woman) when comparison is based on body weight; when comparison is based on body surface area, the rat dose is about 17 times the human dose and the rabbit dose is about 38 times the human dose. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, Imdur tablets should be used during pregnancy only if clearly needed.
- Neonatal survival and development and incidence of stillbirths were adversely affected when pregnant rats were administered oral doses of 750 (but not 300) mg isosorbide mononitrate/kg/day during late gestation and lactation. This dose (about 312 times the human dose when comparison is based on body weight and 54 times the human dose when comparison is based on body surface area) was associated with decreases in maternal weight gain and motor activity and evidence of impaired lactation.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Isosorbide mononitrate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Isosorbide mononitrate during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when ISMN is administered to a nursing mother.
Pediatric Use
- The safety and effectiveness of ISMN in pediatric patients have not been established.
Geriatic Use
- Clinical studies of Imdur tablets did not include sufficient information on patients age 65 and over to determine whether they respond differently from younger patients. Other reported clinical experience for Imdur has not identified differences in response between elderly and younger patients. Clinical experience for organic nitrates reported in the literature identified a potential for severe hypotension and increased sensitivity to nitrates in the elderly. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
- Elderly patients may have reduced baroreceptor function and may develop severe orthostatic hypotension when vasodilators are used. Imdur should therefore be used with caution in elderly patients who may be volume depleted, on multiple medications or who, for whatever reason, are already hypotensive. Hypotension induced by isosorbide mononitrate may be accompanied by paradoxical bradycardia and increased angina pectoris.
- Elderly patients may be more susceptible to hypotension and may be at a greater risk of falling at therapeutic doses of nitroglycerin.
- Nitrate therapy may aggravate the angina caused by hypertrophic cardiomyopathy, particularly in the elderly.
Gender
There is no FDA guidance on the use of Isosorbide mononitrate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Isosorbide mononitrate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Isosorbide mononitrate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Isosorbide mononitrate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Isosorbide mononitrate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Isosorbide mononitrate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Imdur Extended Release Tablets should not be chewed or crushed and should be swallowed together with a half-glassful of fluid. Do not break the 30 mg tablet.
Monitoring
- The benefits of ISMN in patients with acute myocardial infarction or congestive heart failure have not been established; because the effects of isosorbide mononitrate are difficult to terminate rapidly, this drug is not recommended in these settings. If isosorbide mononitrate is used in these conditions, careful clinical or hemodynamic monitoring must be used to avoid the hazards of hypotension and tachycardia.
IV Compatibility
There is limited information regarding IV Compatibility of Isosorbide mononitrate in the drug label.
Overdosage
Hemodynamic Effects
- The ill effects of isosorbide mononitrate overdose are generally the result of isosorbide mononitrate's capacity to induce vasodilatation, venous pooling, reduced cardiac output, and hypotension. These hemodynamic changes may have protean manifestations, including increased intracranial pressure, with any or all of persistent throbbing headache, confusion, and moderate fever; vertigo, palpitations; visual disturbances; nausea and vomiting (possibly with colic and even bloody diarrhea); syncope (especially in the upright posture); air hunger and dyspnea, later followed by reduced ventilatory effort; diaphoresis, with the skin either flushed or cold and clammy; heart block and bradycardia; paralysis; coma; seizures and death.
- Laboratory determinations of serum levels of isosorbide mononitrate and its metabolites are not widely available, and such determinations have, in any event, no established role in the management of isosorbide mononitrate overdose.
- There are no data suggesting what dose of isosorbide mononitrate is likely to be life threatening in humans. In rats and mice, there is significant lethality at doses of 2000 mg/kg and 3000 mg/kg, respectively.
- No data are available to suggest physiological maneuvers (eg, maneuvers to change the pH of the urine) that might accelerate elimination of isosorbide mononitrate. In particular, dialysis is known to be ineffective in removing isosorbide mononitrate from the body.
- No specific antagonist to the vasodilator effects of isosorbide mononitrate is known, and no intervention has been subject to controlled study as a therapy of isosorbide mononitrate overdose. Because the hypotension associated with isosorbide mononitrate overdose is the result of venodilatation and arterial hypovolemia, prudent therapy in this situation should be directed toward an increase in central fluid volume. Passive elevation of the patient's legs may be sufficient, but intravenous infusion of normal saline or similar fluid may also be necessary.
- The use of epinephrine or other arterial vasoconstrictors in this setting is likely to do more harm than good.
- In patients with renal disease or congestive heart failure, therapy resulting in central volume expansion is not without hazard. Treatment of isosorbide mononitrate overdose in these patients may be subtle and difficult, and invasive monitoring may be required.
Methemoglobinemia
- Methemoglobinemia has been reported in patients receiving other organic nitrates, and it probably could also occur as a side effect of isosorbide mononitrate. Certainly nitrate ions liberated during metabolism of isosorbide mononitrate can oxidize hemoglobin into methemoglobin. Even in patients totally without cytochrome b5 reductase activity, however, and even assuming that the nitrate moiety of isosorbide mononitrate is quantitatively applied to oxidation of hemoglobin, about 2 mg/kg of isosorbide mononitrate should be required before any of these patients manifest clinically significant (≥10%) methemoglobinemia. In patients with normal reductase function, significant production of methemoglobin should require even larger doses of isosorbide mononitrate. In one study in which 36 patients received 2-4 weeks of continuous nitroglycerin therapy at 3.1 to 4.4 mg/hr (equivalent, in total administered dose of nitrate ions, to 7.8-11.1 mg of isosorbide mononitrate per hour), the average methemoglobin level measured was 0.2%; this was comparable to that observed in parallel patients who received placebo.
- Notwithstanding these observations, there are case reports of significant methemoglobinemia in association with moderate overdoses of organic nitrates. None of the affected patients had been thought to be unusually susceptible.
- Methemoglobin levels are available from most clinical laboratories. The diagnosis should be suspected in patients who exhibit signs of impaired oxygen delivery despite adequate cardiac output and adequate arterial pO2. Classically, methemoglobinemic blood is described as chocolate brown without color change on exposure to air. When methemoglobinemia is diagnosed, the treatment of choice is methylene blue, 1-2 mg/kg intravenously.
Pharmacology
| |
Isosorbide mononitrate
| |
| Systematic (IUPAC) name | |
| 8-nitrooxy-2,6-dioxabicyclo[3.3.0]octan-4-ol | |
| Identifiers | |
| CAS number | |
| ATC code | C01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 191.139 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | >95% |
| Protein binding | <5% |
| Metabolism | Hepatic |
| Half life | 5 hours |
| Excretion | Renal: 93% |
| Therapeutic considerations | |
| Pregnancy cat. |
C (USA) |
| Legal status | |
| Routes | Oral |
Mechanism of Action
- The Imdur product is an oral extended-release formulation of ISMN, the major active metabolite of isosorbide dinitrate; most of the clinical activity of the dinitrate is attributable to the mononitrate.
- The principal pharmacological action of ISMN and all organic nitrates in general is relaxation of vascular smooth muscle, producing dilatation of peripheral arteries and veins, especially the latter. Dilatation of the veins promotes peripheral pooling of blood, decreases venous return to the heart, thereby reducing left ventricular end-diastolic pressure and pulmonary capillary wedge pressure (preload). Arteriolar relaxation reduces systemic vascular resistance, systolic arterial pressure and mean arterial pressure (afterload). Dilatation of the coronary arteries also occurs. The relative importance of preload reduction, afterload reduction, and coronary dilatation remains undefined.
Structure
- Isosorbide mononitrate (ISMN), an organic nitrate and the major biologically active metabolite of isosorbide dinitrate (ISDN), is a vasodilator with effects on both arteries and veins.
- Imdur® Tablets, for oral administration, contain either 30 mg, 60 mg or 120 mg of isosorbide mononitrate in an extended-release formulation. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, hydrogenated castor oil, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose and talc.
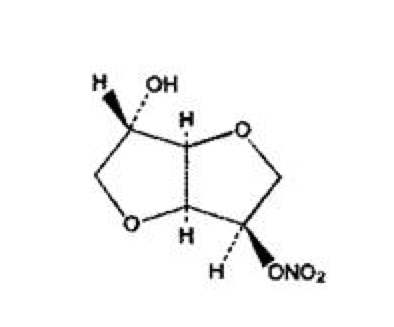
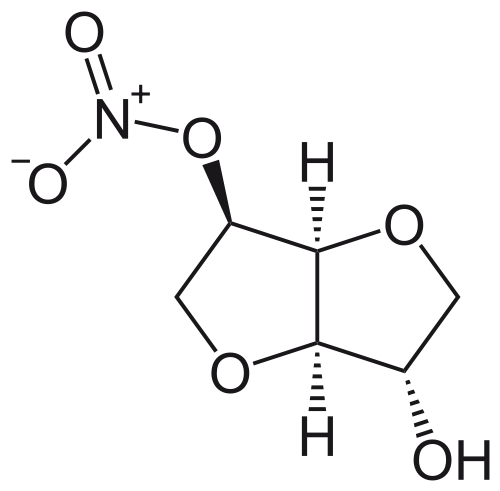
- The molecular formula of ISMN is C6H9NO6 and the molecular weight is 191.14. The chemical name for ISMN is 1,4:3,6-dianhydro-,D-glucitol 5-nitrate; the compound has the following structural formula:
- ISMN is a white, crystalline, odorless compound which is stable in air and in solution, has a melting point of about 90°C, and an optical rotation of +144° (2% in water, 20°C).
- Isosorbide mononitrate is freely soluble in water, ethanol, methanol, chloroform, ethyl acetate, and dichloromethane.
Pharmacodynamics
- Dosing regimens for most chronically used drugs are designed to provide plasma concentrations that are continuously greater than a minimally effective concentration. This strategy is inappropriate for organic nitrates. Several well-controlled clinical trials have used exercise testing to assess the antianginal efficacy of continuously delivered nitrates. In the large majority of these trials, active agents were indistinguishable from placebo after 24 hours (or less) of continuous therapy. Attempts to overcome tolerance by dose escalation, even to doses far in excess of those used acutely, have consistently failed. Only after nitrates have been absent from the body for several hours has their antianginal efficacy been restored. Imdur tablets, during long-term use over 42 days dosed at 120 mg once daily, continued to improve exercise performance at 4 hours and at 12 hours after dosing but its effects (although better than placebo) are less than or at best equal to the effects of the first dose of 60 mg.
Pharmacokinetics
- After oral administration of ISMN as a solution or immediate-release tablets, maximum plasma concentrations of ISMN are achieved in 30 to 60 minutes, with an absolute bioavailability of approximately 100%. After intravenous administration, ISMN is distributed into total body water in about 9 minutes with a volume of distribution of approximately 0.6-0.7 L/kg. Isosorbide mononitrate is approximately 5% bound to human plasma proteins and is distributed into blood cells and saliva. Isosorbide mononitrate is primarily metabolized by the liver, but unlike oral isosorbide dinitrate, it is not subject to first-pass metabolism. Isosorbide mononitrate is cleared by denitration to isosorbide and glucuronidation as the mononitrate, with 96% of the administered dose excreted in the urine within 5 days and only about 1% eliminated in the feces. At least six different compounds have been detected in urine, with about 2% of the dose excreted as the unchanged drug and at least five metabolites. The metabolites are not pharmacologically active. Renal clearance accounts for only about 4% of total body clearance. The mean plasma elimination half-life of ISMN is approximately 5 hours.
- The disposition of ISMN in patients with various degrees of renal insufficiency, liver cirrhosis, or cardiac dysfunction was evaluated and found to be similar to that observed in healthy subjects. The elimination half-life of ISMN was not prolonged, and there was no drug accumulation in patients with chronic renal failure after multiple oral dosing.
- The pharmacokinetics and/or bioavailability of Imdur tablets have been studied in both normal volunteers and patients following single- and multiple-dose administration. Data from these studies suggest that the pharmacokinetics of ISMN administered as Imdur tablets are similar between normal healthy volunteers and patients with angina pectoris. In single- and multiple-dose studies, the pharmacokinetics of ISMN were dose proportional between 30 mg and 240 mg.
- In a multiple-dose study, the effect of age on the pharmacokinetic profile of Imdur 60 mg and 120 mg (2 × 60 mg) was evaluated in subjects ≥45 years. The results of that study indicate that there are no significant differences in any of the pharmacokinetic variables of ISMN between elderly (≥65 years) and younger individuals (45–64 years) for the Imdur 60 mg dose. The administration of Imdur tablets 120 mg (2 × 60 mg tablets every 24 hours for 7 days) produced a dose-proportional increase in Cmax and AUC, without changes in Tmax or the terminal half-life. The older group (65-74 years) showed 30% lower apparent oral clearance (Cl/F) following the higher dose, i.e., 120 mg, compared to the younger group (45-64 years); Cl/F was not different between the two groups following the 60 mg regimen. While Cl/F was independent of dose in the younger group, the older group showed slightly lower Cl/F following the 120 mg regimen compared to the 60 mg regimen. Differences between the two age groups, however, were not statistically significant. In the same study, females showed a slight (15%) reduction in clearance when the dose was increased. Females showed higher AUCs and Cmax compared to males, but these differences were accounted for by differences in body weight between the two groups. When the data were analyzed using age as a variable, the results indicated that there were no significant differences in any of the pharmacokinetic variables of ISMN between older (≥65 years) and younger individuals (45-64 years). The results of this study, however, should be viewed with caution due to the small number of subjects in each age subgroup and consequently the lack of sufficient statistical power.
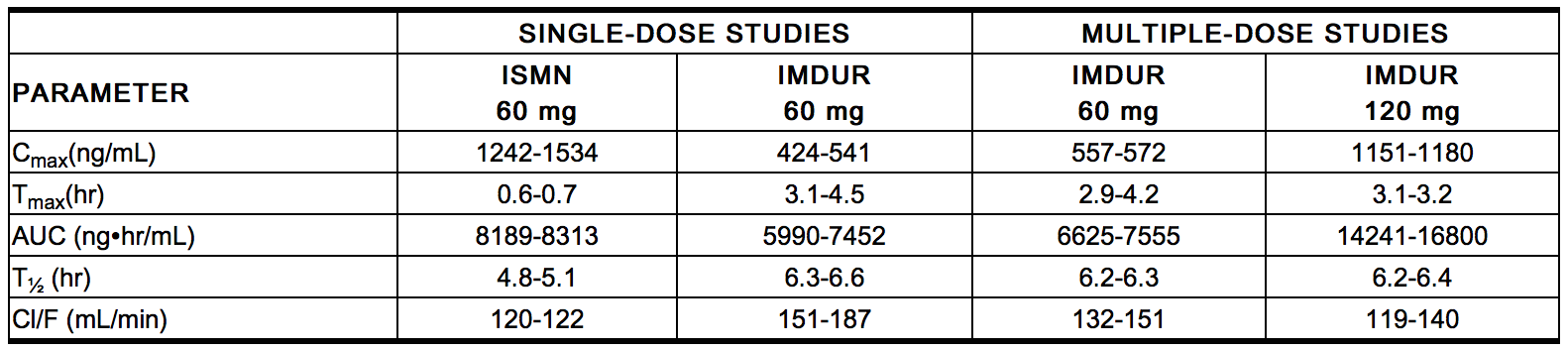
- The following table summarizes key pharmacokinetic parameters of ISMN after single- and multiple-dose administration of ISMN as an oral solution or Imdur tablets:
- Food Effects
- The influence of food on the bioavailability of ISMN after single-dose administration of Imdur tablets 60 mg was evaluated in three different studies involving either a "light" breakfast or a high-calorie, high-fat breakfast. Results of these studies indicate that concomitant food intake may decrease the rate (increase in Tmax) but not the extent (AUC) of absorption of ISMN.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- No evidence of carcinogenicity was observed in rats exposed to isosorbide mononitrate in their diets at doses of up to 900 mg/kg/day for the first 6 months and 500 mg/kg/day for the remaining duration of a study in which males were dosed for up to 121 weeks and females were dosed for up to 137 weeks. No evidence of carcinogenicity was observed in mice exposed to isosorbide mononitrate in their diets for up to 104 weeks at doses of up to 900 mg/kg/day.
- Isosorbide mononitrate did not produce gene mutations (Ames test, mouse lymphoma test) or chromosome aberrations (human lymphocyte and mouse micronucleus tests) at biologically relevant concentrations.
- No effects on fertility were observed in a study in which male and female rats were administered doses of up to 750 mg/kg/day beginning, in males, 9 weeks prior to mating, and in females, 2 weeks prior to mating.
Clinical Studies
- Controlled trials with Imdur tablets have demonstrated antianginal activity following acute and chronic dosing. Administration of Imdur tablets once daily, taken early in the morning on arising, provided at least 12 hours of antianginal activity.
- In a placebo-controlled parallel study, 30, 60, 120 and 240 mg of Imdur tablets were administered once daily for up to 6 weeks. Prior to randomization, all patients completed a 1- to 3-week single-blind placebo phase to demonstrate nitrate responsiveness and total exercise treadmill time reproducibility. Exercise tolerance tests using the Bruce Protocol were conducted prior to and at 4 and 12 hours after the morning dose on days 1, 7, 14, 28 and 42 of the double-blind period. Imdur tablets 30 and 60 mg (only doses evaluated acutely) demonstrated a significant increase from baseline in total treadmill time relative to placebo at 4 and 12 hours after the administration of the first dose. At day 42, the 120 and 240 mg dose of Imdur tablets demonstrated a significant increase in total treadmill time at 4 and 12 hours post dosing, but by day 42, the 30 and 60 mg doses no longer were differentiable from placebo. Throughout chronic dosing, rebound was not observed in any Imdur treatment group.
- Pooled data from two other trials, comparing Imdur tablets 60 mg once daily, ISDN 30 mg QID, and placebo QID in patients with chronic stable angina using a randomized, double-blind, three-way crossover design found statistically significant increases in exercise tolerance times for Imdur tablets compared to placebo at hours 4, 8 and 12 and to ISDN at hour 4. The increases in exercise tolerance on day 14, although statistically significant compared to placebo, were about half of that seen on day 1 of the trial.
How Supplied
- Imdur Extended Release Tablets 30 mg are white, capsule-shaped tablets scored on one side and engraved "Imdur" on the unscored side. They are supplied as follows:
Storage
There is limited information regarding Isosorbide mononitrate Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Isosorbide mononitrate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Isosorbide mononitrate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should be told that the antianginal efficacy of Imdur tablets can be maintained by carefully following the prescribed schedule of dosing. For most patients, this can be accomplished by taking the dose on arising.
- As with other nitrates, daily headaches sometimes accompany treatment with isosorbide mononitrate. In patients who get these headaches, the headaches are a marker of the activity of the drug. Patients should resist the temptation to avoid headaches by altering the schedule of their treatment with isosorbide mononitrate, since loss of headache may be associated with simultaneous loss of antianginal efficacy. Aspirin or acetaminophen often successfully relieves isosorbide mononitrate-induced headaches with no deleterious effect on isosorbide mononitrate's antianginal efficacy.
- Treatment with isosorbide mononitrate may be associated with lightheadedness on standing, especially just after rising from a recumbent or seated position. This effect may be more frequent in patients who have also consumed alcohol.
Precautions with Alcohol
- The vasodilating effects of isosorbide mononitrate may be additive with those of other vasodilators. Alcohol, in particular, has been found to exhibit additive effects of this variety.
Brand Names
- Imdur®[2]
Look-Alike Drug Names
- N/A[3]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Merkel, C. (1996-12-21). "Randomised trial of nadolol alone or with isosorbide mononitrate for primary prophylaxis of variceal bleeding in cirrhosis. Gruppo-Triveneto per L'ipertensione portale (GTIP)". Lancet. 348 (9043): 1677–1681. ISSN 0140-6736. PMID 8973428. Unknown parameter
|coauthors=ignored (help) - ↑ "Imdur (isosorbide mononitrate) tablet, extended release".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Isosorbide mononitrate |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Isosorbide mononitrate |Label Name=Isosorbide mononitrate05.png
}}
{{#subobject:
|Label Page=Isosorbide mononitrate |Label Name=Isosorbide mononitrate06.png
}}
{{#subobject:
|Label Page=Isosorbide mononitrate |Label Name=Isosorbide mononitrate07.png
}}
{{#subobject:
|Label Page=Isosorbide mononitrate |Label Name=Isosorbide mononitrate08.png
}}