Estropipate (vaginal)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Title
See full prescribing information for complete Boxed Warning.
WARNING:
ESTROGENS HAVE BEEN REPORTED TO INCREASE THE RISK OF ENDOMETRIAL CARClNOMA IN POSTMENOPAUSAL WOMEN
ESTROGENS SHOULD NOT BE USED DURING PREGNANCY
|
Overview
Estropipate (vaginal) is an endocrine-metabolic agent that is FDA approved for the treatment of vulval and vaginal atrophy. There is a Black Box Warning for this drug as shown here. Common adverse reactions include Edema, Chloasma, Hirsutism, Bloating, Nausea, Stomach cramps, Vomiting, Headache, Migraine, Depression, Breast tenderness, Disorder of menstruation.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- EstropipateVaginal Cream is indicated for the treatment of vulval and vaginal atrophy.
Dosage
- For treatment of vulval and vaginal atrophy associated with the menopause, the lowest dose and regimen that will control symptoms should be chosen and medication should be discontinued as promptly as possible.
- Attempts to discontinue or taper medication should be made at 3-month to 6-month intervals.
- Usual dosage range
- Intravaginally, 2 to 4 grams of Estropipate Vaginal Cream daily, depending upon the severity of the condition.
- The following instructions for use are intended for the patient and are printed on the carton label for EstropipateVaginal Cream (estropipate):
- Remove cap from tube.
- Make sure plunger of applicator is all the way into the barrel.
- Screw nozzle end of applicator onto the tube.
- Squeeze tube to force sufficient cream into applicator so that number on plunger indicating prescribed dose is level with top of barrel.
- Unscrew applicator from tube and replace cap on tube.
- To deliver medication, insert end of applicator into vagina and push plunger all the way down.
- Between uses, pull plunger out of barrel and wash applicator in warm, soapy water. DO NOT PUT APPLICATOR IN HOT OR BOILING WATER.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Estropipate (vaginal) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Estropipate (vaginal) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Estropipate (vaginal) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Estropipate (vaginal) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Estropipate (vaginal) in pediatric patients.
Contraindications
- Estrogens should not be used in individuals with any of the following conditions
- Known or suspected pregnancy . Estrogens may cause fetal harm when administered to a pregnant woman
- Undiagnosed abnormal genital bleeding.
- Known or suspected cancer of the breast except in appropriately selected patients being treated for metastatic disease.
- Known or suspected estrogen-dependent neoplasia.
- Active thrombophlebitis or thromboembolic disorders.
- EstropipateVaginal Cream (estropipate) is contraindicated in patients hypersensitive to its ingredients.
Warnings
|
Title
See full prescribing information for complete Boxed Warning.
WARNING:
ESTROGENS HAVE BEEN REPORTED TO INCREASE THE RISK OF ENDOMETRIAL CARClNOMA IN POSTMENOPAUSAL WOMEN
ESTROGENS SHOULD NOT BE USED DURING PREGNANCY
|
- Induction of malignant neoplasms
- Endometrial cancer
- The reported endometrial cancer risk among unopposed Estropipateusers is about 2 to 12 fold greater than in non-users, and appears dependent on duration of treatment and on Estropipate dose. Most studies show no significant increased risk associated with use of estrogens for less than one year. The greatest risk appears associated with prolonged use – with increased risks of 15 to 24-fold for five to ten years or more. In three studies, persistence of risk was demonstrated for 8 to over 15 years after cessation of Estropipate treatment. In one study a significant decrease in the incidence of endometrial cancer occurred six months after Estropipate withdrawal. Concurrent progestin therapy may offset this risk but the overall health impact in postmenopausal women is not known .
- Breast Cancer
- While the majority of studies have not shown an increased risk of breast cancer in women who have ever used Estropipate replacement therapy, some have reported a moderately increased risk (relative risks of 1.3–2.0) in those taking higher doses or those taking lower doses for prolonged periods of time, especially in excess of 10 years. Other studies have not shown this relationship.
- Congenital lesions with malignant potential
- Estropipate therapy during pregnancy is associated with an increased risk of fetal congenital reproductive tract disorders, and possibly other birth defects. Studies of women who received DES during pregnancy have shown that female offspring have an increased risk of vaginal adenosis, squamous cell dysplasia of the uterine cervix, and clear cell vaginal cancer later in life; male offspring have an increased risk of urogenital abnormalities and possibly testicular cancer later in life. Although some of these changes are benign, others are precursors of malignancy.
- Gallbladder disease
- Two studies have reported a 2- to 4-fold increase in the risk of gallbladder disease requiring surgery in women receiving postmenopausal estrogens.
- Cardiovascular disease
- Large doses of Estropipate(5 mg conjugated estrogens per day), comparable to those used to treat cancer of the prostate and breast, have been shown in a large prospective clinical trial in men to increase the risks of nonfatal myocardial infarction, pulmonary embolism, and thrombophlebitis. These risks cannot necessarily be extrapolated from men to women. However, to avoid the theoretical cardiovascular risk to women caused by high Estropipatedoses, the dose for Estropipate replacement therapy should not exceed the lowest effective dose.
- Elevated blood pressure
- Occasional blood pressure increases during Estropipate replacement therapy have been attributed to idiosyncratic reactions to estrogens. More often, blood pressure has remained the same or has dropped. One study showed that postmenopausal Estropipate users have higher blood pressure than nonusers. Two other studies showed slightly lower blood pressure among Estropipateusers compared to nonusers. Postmenopausal Estropipate use does not increase the risk of stroke. Nonetheless, blood pressure should be monitored at regular intervals with Estropipate use.
- Hypercalcemia
- Administration of estrogens may lead to severe hypercalcemia in patients with breast cancer and bone metastases. If this occurs, the drug should be stopped and appropriate measures taken to reduce the serum calcium level.
Precautions
- Addition of a progestin
- Studies of the addition of a progestin for seven or more days of a cycle of Estropipateadministration have reported a lowered incidence of endometrial hyperplasia which would otherwise be induced by Estropipate treatment. Morphological and biochemical studies of endometrium suggest that 10 to 14 days of progestin are needed to provide maximal maturation of the endometrium and to eliminate any hyperplastic changes. There are possible additional risks which may be associated with the inclusion of progestins in Estropipate replacement regimens. These include: (1) adverse effects on lipoprotein metabolism (lowering HDL and raising LDL) which may diminish the possible cardioprotective effect of Estropipate therapy ; (2) impairment of glucose tolerance; and (3) possible enhancement of mitotic activity in breast epithelial tissue (although few epidemiological data are available to address this point). The choice of progestin, its dose, and its regimen may be important in minimizing these adverse effects, but these issues remain to be clarified.
- Physical examination
- A complete medical and family history should be taken prior to the initiation of any Estropipate therapy. The pretreatment and periodic physical examinations should include special reference to blood pressure, breasts, abdomen, and pelvic organs, and should include a Papanicolaou smear. As a general rule, Estropipate should not be prescribed for longer than one year without reexamining the patient.
- Hypercoagulability
- Some studies have shown that women taking Estropipate replacement therapy have hypercoagulability, primarily related to decreased antithrombin activity. This effect appears dose- and duration-dependent and is less pronounced than that associated with oral contraceptive use. Also, postmenopausal women tend to have increased coagulation parameters at baseline compared to premenopausal women. There is some suggestion that low dose postmenopausal mestranol may increase the risk of thromboembolism, although the majority of studies (of primarily conjugated estrogens users) report no such increase. There is insufficient information on hypercoagulability in women who have had previous thromboembolic disease.
- Familial hyperlipoproteinemia
- Estropipate therapy may be associated with massive elevations of plasma triglycerides leading to pancreatitis and other complications in patients with familial defects of lipoprotein metabolism.
- Fluid retention
- Uterine bleeding and mastodynia
- Certain patients may develop undesirable manifestations of estrogenic stimulation, such as abnormal uterine bleeding and mastodynia.
- Impaired liver function
- Estropipate may be poorly metabolized in patients with impaired liver function and should be administered with caution.
Adverse Reactions
Clinical Trials Experience
- Hypersensitivity reactions, systemic effects such as breast tenderness, and rarely, withdrawal bleeding, have occurred with the use of topical estrogens. Local irritation (especially when prior inflammation is present) has occurred at initiation of therapy.
- The following additional adverse reactions have been reported with Estropipate therapy (see WARNINGS regarding induction of neoplasia, adverse effects on the fetus, increased incidence of gallbladder disease, cardiovascular disease, elevated blood pressure, and hypercalcemia).
- Genitourinary system.
- Changes in vaginal bleeding pattern and abnormal withdrawal bleeding or flow; breakthrough bleeding, spotting.
- Increase in size of uterine leiomyomata.
- Vaginal candidiasis.
- Change in amount of cervical secretion.
- Breasts.
- Tenderness, enlargement.
- Gastrointestinal.
- Nausea, vomiting.
- Abdominal cramps, bloating.
- Cholestatic jaundice.
- Increased incidence of gallbladder disease.
- Skin.
- Chloasma or melasma which may persist when drug is discontinued.
- Erythema multiforme.
- Erythema nodosum.
- Hemorrhagic eruption.
- Loss of scalp hair.
- Hirsutism.
- Eyes.
- Steepening of corneal curvature.
- Intolerance to contact lenses.
- Central Nervous System.
- Headache, migraine, dizziness.
- Mental depression.
- Chorea.
- Miscellaneous.
- Genitourinary system.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Estropipate (vaginal) in the drug label.
Drug Interactions
- Accelerated prothrombin time, partial thromboplastin time, and platelet aggregation time; increased platelet count; increased factors II, VII antigen, VIII antigen, VIII coagulant activity, IX, X, XII, VII—X complex, II—VII—X complex, and beta-thromboglobulin; decreased levels of anti-factor Xa and antithrombin III, decreased antithrombin III activity; increased levels of fibrin Estropipate and fibrin Estropipate activity; increased plasmin Estropipate antigen and activity.
- Increased thyroid-binding globulin (TBG) leading to increased circulating total thyroid hormone, as measured by protein-bound iodine (PBI), T4 levels (by column or by radioimmunoassay) or T3 levels by radioimmunoassay. T3 resin uptake is decreased, reflecting the elevated TBG. Free T4 and free T3 concentrations are unaltered.
- Other binding proteins may be elevated in serum, i.e., corticosteroid binding globulin (CBG), sex hormone-binding globulin (SHBG), leading to increased circulating corticosteroids and sex steroids respectively. Free or biologically active hormone concentrations are unchanged. Other plasma proteins may be increased (angiotensinogen/renin substrate, alpha-l-antitrypsin, ceruloplasmin).
- Increased plasma HDL and HDL-2 subfraction concentrations, reduced LDL cholesterol concentration, increased triglycerides levels.
- Impaired glucose tolerance.
- Reduced response to metyrapone test.
- Reduced serum folate concentration.
Use in Specific Populations
Pregnancy
- Estrogens should not be used during pregnancy.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Estropipate (vaginal) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Estropipate (vaginal) during labor and delivery.
Nursing Mothers
- As a general principle, the administration of any drug to nursing mothers should be done only when clearly necessary since many drugs are excreted in human milk. In addition, estrEstropipateadministration to nursing mothers has been shown to decrease the quantity and quality of the milk.
Pediatric Use
There is no FDA guidance on the use of Estropipate (vaginal) with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Estropipate (vaginal) with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Estropipate (vaginal) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Estropipate (vaginal) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Estropipate (vaginal) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Estropipate (vaginal) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Estropipate (vaginal) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Estropipate (vaginal) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Vaginal
Monitoring
There is limited information regarding Monitoring of Estropipate (vaginal) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Estropipate (vaginal) in the drug label.
Overdosage
- Serious ill effects have not been reported following acute ingestion of large doses of estrogen-containing oral contraceptives by young children. Overdosage of Estropipatemay cause nausea and vomiting, and withdrawal bleeding may occur in females.
Pharmacology
| |
Estropipate (vaginal)
| |
| Systematic (IUPAC) name | |
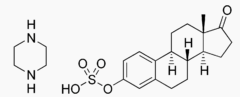
| [(8R,9S,13S,14S)-13-Methyl-17-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-3-yl] hydrEstropipatesulfate; piperazine | |
| Identifiers | |
| CAS number | |
| ATC code | none |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 436.56 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- Estropipate drug products act by regulating the transcription of a limited number of genes. Estrogens diffuse through cell membranes, distribute themselves throughout the cell, and bind to and activate the nuclear estrEstropipatereceptor, a DNA-binding protein which is found in estrogen-responsive tissues. The activated Estropipate receptor binds to specific DNA sequences, or hormone-response elements, which enhance the transcription of adjacent genes and in turn lead to the observed effects. Estropipate receptors have been identified in tissues of the reproductive tract, breast, pituitary, hypothalamus, liver, and bone of women.
- Estrogens are important in the development and maintenance of the female reproductive system and secondary sex characteristics. By a direct action, they cause growth and development of the uterus, fallopian tubes, and vagina. With other hormones, such as pituitary hormones and progesterone, they cause enlargement of the breasts through promotion of ductal growth, stromal development, and the accretion of fat.
- Estrogens are intricately involved with other hormones, especially progesterone, in the processes of the ovulatory menstrual cycle and pregnancy, and affect the release of pituitary gonadotropins. They also contribute to the shaping of the skeleton, maintenance of tone and elasticity of urogenital structures, changes in the epiphyses of the long bones that allow for the pubertal growth spurt and its termination, and pigmentation of the nipples and genitals.
- Estrogens occur naturally in several forms. The primary source of estrEstropipatein normally cycling adult women is the ovarian follicle, which secretes 70 to 500 micrograms of estradiol daily, depending on the phase of the menstrual cycle. This is converted primarily to estrone, which circulates in roughly equal proportion to estradiol, and to small amounts of estriol. After menopause, most endogenous Estropipate is produced by conversion of androstenedione, secreted by the adrenal cortex, to estrone by peripheral tissues. Thus, estrone – especially in its sulfate ester form – is the most abundant circulating Estropipate in postmenopausal women. Although circulating estrogens exist in a dynamic equilibrium of metabolic interconversions, estradiol is the principal intracellular human estrEstropipate and is substantially more potent than estrone or estriol at the receptor.
- Estrogens used in therapy are well absorbed through the skin, mucous membranes, and gastrointestinal tract. When applied for a local action, absorption is usually sufficient to cause systemic effects. When conjugated with aryl and alkyl groups for parenteral administration, the rate of absorption of oily preparations is slowed with a prolonged duration of action, such that a single intramuscular injection of estradiol valerate or estradiol cypionate is absorbed over several weeks.
Structure
- Estropipate(estropipate vaginal cream, USP), (formerly piperazine estrone sulfate), is a natural estrogenic substance prepared from purified crystalline estrone, solubilized as the sulfate and stabilized with piperazine. It is appreciably soluble in water and has almost no odor or taste. The amount of piperazine in Estropipate is not sufficient to exert a pharmacological action. Its addition ensures solubility, stability, and uniform potency of the estrone sulfate. Chemically estropipate, molecular weight: 436.56, is represented by estra-1,3,5(10)-trien-17-one,3-(sulfooxy)-, compound with piperazine (1:1). The structural formula may be represented as follows:
- Each gram of Estropipate Vaginal Cream contains 1.5 mg estropipate in a base composed of the following ingredients: glycerin, mineral oil, glyceryl monostearate, polyethylene glycol ether complex of higher fatty alcohols, cetyl alcohol, anhydrous lanolin, sodium biphosphate, cis-N-(3-chloroallyl) hexaminium chloride, propylparaben, methylparaben, piperazine hexahydrate, citric acid and water.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Estropipate (vaginal) in the drug label.
Pharmacokinetics
- Administered estrogens and their esters are handled within the body essentially the same as the endogenous hormones. Metabolic conversion of estrogens occurs primarily in the liver (first pass effect), but also at local target tissue sites. Complex metabolic processes result in a dynamic equilibrium of circulating conjugated and unconjugated estrogenic forms which are continually interconverted, especially between estrone and estradiol and between esterified and unesterified forms. Although naturally-occurring estrogens circulate in the blood largely bound to sex hormone-binding globulin and albumin, only unbound estrogens enter target tissue cells. A significant proportion of the circulating estrEstropipateexists as sulfate conjugates, especially estrone sulfate, which serves as a circulating reservoir for the formation of more active estrogenic species. A certain proportion of the estrEstropipateis excreted into the bile and then reabsorbed from the intestine. During this enterohepatic recirculation, estrogens are desulfated and resulfated and undergo degradation through conversion to less active estrogens (estriol and other estrogens), oxidation to nonestrogenic substances (catecholestrogens, which interact with catecholamine metabolism, especially in the central nervous system), and conjugation with glucuronic acids (which are then rapidly excreted in the urine).
- When given orally, naturally-occurring estrogens and their esters are extensively metabolized (first pass effect) and circulate primarily as estrone sulfate, with smaller amounts of other conjugated and unconjugated estrogenic species. This results in limited oral potency. By contrast, synthetic estrogens, such as ethinyl estradiol and the nonsteroidal estrogens, are degraded very slowly in the liver and other tissues, which results in their high intrinsic potency. Estropipatedrug products administered by non-oral routes are not subject to first-pass metabolism, but also undergo significant hepatic uptake, metabolism, and enterohepatic recycling.
Nonclinical Toxicology
- Carcinogenesis, Mutagenesis, and Impairment of Fertility
- Long-term continuous administration of natural and synthetic estrogens in certain animal species increases the frequency of carcinomas of the breast, uterus, cervix, vagina, testis, and liver.
Clinical Studies
There is limited information regarding Clinical Studies of Estropipate (vaginal) in the drug label.
How Supplied
- Estropipate(estropipate vaginal cream, USP), 1.5 mg estropipate per gram, is available in packages containing a 1½ oz (42.5 g) tube with one plastic applicator calibrated at 1, 2, 3, and 4 g levels, (NDC 0009-3776-01).
- Store below 86° F (30° C).
Storage
There is limited information regarding Estropipate (vaginal) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Estropipate (vaginal) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Estropipate (vaginal) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Estropipate (vaginal) in the drug label.
Precautions with Alcohol
- Alcohol-Estropipate (vaginal) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- OGEN
Look-Alike Drug Names
There is limited information regarding Estropipate (vaginal) Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Estropipate (vaginal) |Label Name=Estropipate (vaginal)03.png
}}
