Dopamine
| |
| |
| Names | |
|---|---|
| IUPAC name
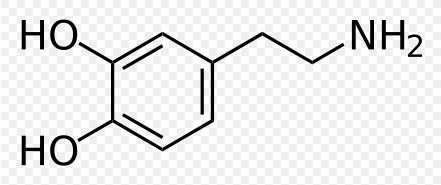
4-(2-aminoethyl)benzene-1,2-diol
| |
| Other names
2-(3,4-dihydroxyphenyl)ethylamine;
3,4-dihydroxyphenethylamine; 3-hydroxytyramine; DA; Intropin; Revivan; Oxytyramine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H11NO2 | |
| Molar mass | 153.18 g/mol |
| Hazards | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
|
WikiDoc Resources for Dopamine |
|
Articles |
|---|
|
Most recent articles on Dopamine |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Dopamine at Clinical Trials.gov Clinical Trials on Dopamine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Dopamine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating Dopamine Risk calculators and risk factors for Dopamine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Dopamine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [5]
Overview
Dopamine is a catecholamine neurotransmitter present in a wide variety of animals, including both vertebrates and invertebrates. In the brain, this phenethylamine functions as a neurotransmitter, activating the five types of dopamine receptors—D1, D2, D3, D4, and D5—and their variants. Dopamine is produced in several areas of the brain, including the substantia nigra and the ventral tegmental area.[1] Dopamine is also a neurohormone released by the hypothalamus. Its main function as a hormone is to inhibit the release of prolactin from the anterior lobe of the pituitary.
Dopamine is available as an intravenous medication acting on the sympathetic nervous system, producing effects such as increased heart rate and blood pressure. However, because dopamine cannot cross the blood-brain barrier, dopamine given as a drug does not directly affect the central nervous system. To increase the amount of dopamine in the brains of patients with diseases such as Parkinson's disease and dopa-responsive dystonia, L-DOPA, which is the precursor of dopamine, can be given because it can cross the blood-brain barrier.
History
Dopamine was first synthesized in 1910 by George Barger and James Ewens at Wellcome Laboratories in London, England.[2] It was named dopamine because it was a monoamine, and its synthetic precursor was 3,4-dihydroxyphenylalanine (L-DOPA). Dopamine's function as a neurotransmitter was first recognized in 1958 by Arvid Carlsson and Nils-Åke Hillarp at the Laboratory for Chemical Pharmacology of the National Heart Institute of Sweden.[3] Carlsson was awarded the 2000 Nobel Prize in Physiology or Medicine for showing that dopamine is not just a precursor of norepinephrine (noradrenaline) and epinephrine (adrenaline), but a neurotransmitter as well.
Biochemistry
Name and family
Dopamine has the chemical formula C6H3(OH)2-CH2-CH2-NH2. Its chemical name is "4-(2-aminoethyl)benzene-1,2-diol" and its abbreviation is "DA."
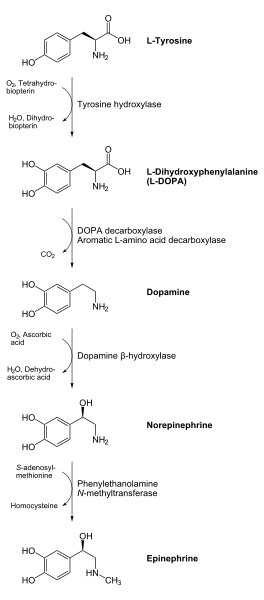
As a member of the catecholamine family, dopamine is a precursor to norepinephrine (noradrenaline) and then epinephrine (adrenaline) in the biosynthetic pathways for these neurotransmitters.
Biosynthesis
Dopamine is biosynthesized in the body (mainly by nervous tissue and the medulla of the adrenal glands) first by the hydroxylation of the amino acid L-tyrosine to L-DOPA via the enzyme tyrosine 3-monooxygenase, also known as tyrosine hydroxylase, and then by the decarboxylation of L-DOPA by aromatic L-amino acid decarboxylase (which is often referred to as dopa decarboxylase). In some neurons, dopamine is further processed into norepinephrine by dopamine beta-hydroxylase.
In neurons, dopamine is packaged after synthesis into vesicles, which are then released into the synapse in response to a presynaptic action potential.
Inactivation and degradation
Two major degradation pathways for dopamine exist. In most areas of the brain, including the striatum and basal ganglia, dopamine is inactivated by reuptake via the dopamine transporter (DAT1), then enzymatic breakdown by monoamine oxidase (MAOA and MAOB) into 3,4-dihydroxyphenylacetic acid. In the prefrontal cortex, however, there are very few dopamine transporter proteins, and dopamine is instead inactivated by reuptake via the norepinephrine transporter (NET), presumably on neighboring norepinephrine neurons, then enzymatic breakdown by catechol-O-methyl transferase (COMT) into 3-methoxytyramine.[4] The DAT1 pathway is roughly an order of magnitude faster than the NET pathway: in mice, dopamine concentrations decay with a half-life of 200 ms in the caudate nucleus (which uses the DAT1 pathway) versus 2,000 ms in the prefrontal cortex.[5] Dopamine that is not broken down by enzymes is repackaged into vesicles for reuse by VMAT2.
Functions in the brain
Dopamine has many functions in the brain, including important roles in behavior and cognition, voluntary movement, motivation, punishment and reward, inhibition of prolactin production (involved in lactation and sexual gratification), sleep, mood, attention, working memory, and learning. Dopaminergic neurons (i.e., neurons whose primary neurotransmitter is dopamine) are present chiefly in the ventral tegmental area (VTA) of the midbrain, the substantia nigra pars compacta, and the arcuate nucleus of the hypothalamus.
It has been hypothesized that dopamine transmits reward prediction error, although this has been questioned.[6] According to this hypothesis, the phasic responses of dopamine neurons are observed when an unexpected reward is presented. These responses transfer to the onset of a conditioned stimulus after repeated pairings with the reward. Further, dopamine neurons are depressed when the expected reward is omitted. Thus, dopamine neurons seem to encode the prediction error of rewarding outcomes. In nature, we learn to repeat behaviors that lead to maximizing rewards. Dopamine is therefore believed to provide a teaching signal to parts of the brain responsible for acquiring new behavior. Temporal difference learning provides a computational model describing how the prediction error of dopamine neurons is used as a teaching signal.
The reward system in insects uses octopamine, which is the presumed arthropod homolog of norepinephrine,[7] rather than dopamine. In insects, dopamine acts instead as a punishment signal and is necessary to form aversive memories.[8][9]
Anatomy
Dopaminergic neurons form a neurotransmitter system which originates in substantia nigra pars compacta, ventral tegmental area (VTA), and hypothalamus. These project axons to large areas of the brain which are typically divided into four major pathways:
- Mesocortical pathway connects the ventral tegmental area to the frontal lobe of the pre-frontal cortex. Neurons with somas in the ventral tegmental area project axons into the pre-frontal cortex.
- Mesolimbic pathway carries dopamine from the ventral tegmental area to the nucleus accumbens via the amygdala and hippocampus. The somas of the projecting neurons are in the ventral tegmental area.
- Nigrostriatal pathway runs from the substantia nigra to the neostriatum. Somas in the substantia nigra project axons into the caudate nucleus and putamen. The pathway is involved in the basal ganglia motor loop.
- Tuberoinfundibular pathway runs from the hypothalamus to the pituitary gland.
This innervation explains many of the effects of activating this dopamine system. For instance, the mesolimbic pathway connects the VTA and nucleus accumbens; both are central to the brain reward system.[10]
Whilst the distinction between pathways is widely used, and is regarded as a “convenient heuristic when considering the dopamine system”, it is not absolute, and there is some overlap in the projection targets of each group of neurons.[11]
Tonic and phasic activity
The level of extracellular dopamine is modulated by two mechanisms, tonic and phasic dopamine transmission. Tonic dopamine transmission occurs when small amounts of dopamine are released independently of neuronal activity, and is regulated by the activity of other neurons and neurotransmitter reuptake.[12] Phasic dopamine release results from the activity of the dopamine-containing cells themselves. This activity is characterized by irregular pacemaking activity of single spikes, and rapid bursts of typically 2-6 spikes in quick succession.[13][14] Concentrated bursts of activity result in a greater increase of extracellular dopamine levels than would be expected from the same number of spikes distributed over a longer period of time.[15]
Movement
Via the dopamine receptors, D1-5, dopamine reduces the influence of the indirect pathway, and increases the actions of the direct pathway within the basal ganglia. Insufficient dopamine biosynthesis in the dopaminergic neurons can cause Parkinson's disease, in which a person loses the ability to execute smooth, controlled movements.
Cognition and frontal cortex
In the frontal lobes, dopamine controls the flow of information from other areas of the brain. Dopamine disorders in this region of the brain can cause a decline in neurocognitive functions, especially memory, attention, and problem-solving. Reduced dopamine concentrations in the prefrontal cortex are thought to contribute to attention deficit disorder. It has been found that D1 receptors[16] as well as D4 receptors[17] are responsible for the cognitive-enhancing effects of dopamine.
Regulating prolactin secretion
Dopamine is the primary neuroendocrine inhibitor of the secretion of prolactin from the anterior pituitary gland.[18] Dopamine produced by neurons in the arcuate nucleus of the hypothalamus is secreted into the hypothalamo-hypophysial blood vessels of the median eminence, which supply the pituitary gland. The lactotrope cells that produce prolactin, in the absence of dopamine, secrete prolactin continuously; dopamine inhibits this secretion. Thus, in the context of regulating prolactin secretion, dopamine is occasionally called prolactin-inhibiting factor (PIF), prolactin-inhibiting hormone (PIH), or prolactostatin.
Motivation and pleasure
Reinforcement
Dopamine is commonly associated with the reward system of the brain, providing feelings of enjoyment and reinforcement to motivate a person proactively to perform certain activities. Dopamine is released (particularly in areas such as the nucleus accumbens and prefrontal cortex) by rewarding experiences such as food, sex, drugs, and neutral stimuli that become associated with them. Recent studies indicate that aggression may also stimulate the release of dopamine in this way.[19]
This theory is often discussed in terms of drugs such as cocaine, nicotine, and amphetamines, which directly or indirectly lead to an increase of dopamine in the mesolimbic reward pathway of the brain, and in relation to neurobiological theories of chemical addiction (not to be confused with psychological dependence), arguing that this dopamine pathway is pathologically altered in addicted persons.[20][21][22]
Reuptake inhibition, expulsion
Cocaine and amphetamines inhibit the re-uptake of dopamine; however, they influence separate mechanisms of action. Cocaine is a dopamine transporter and norepinephrine transporter blocker that competitively inhibits dopamine uptake to increase the lifetime of dopamine and augments an overabundance of dopamine (an increase of up to 150 percent) within the parameters of the dopamine neurotransmitters. Like cocaine, amphetamines increase the concentration of dopamine in the synaptic gap, but by a different mechanism. Amphetamines and methamphetamine are similar in structure to dopamine, and so can enter the terminal bouton of the presynaptic neuron via its dopamine transporters as well as by diffusing through the neural membrane directly.[citation needed] By entering the presynaptic neuron, amphetamines force dopamine molecules out of their storage vesicles and expel them into the synaptic gap by making the dopamine transporters work in reverse.
Glycine and Taurine has been shown to alter dopamine levels in rat brains. [23] [24] [25]
Incentive salience
Dopamine's role in experiencing pleasure has been questioned by several researchers. It has been argued that dopamine is more associated with anticipatory desire and motivation (commonly referred to as "wanting") as opposed to actual consummatory pleasure (commonly referred to as "liking").
Dopamine, learning, and reward-seeking behavior
Dopaminergic neurons of the midbrain are the main source of dopamine in the brain.[26] Dopamine has been shown to be involved in the control of movements, the signaling of error in prediction of reward, motivation, and cognition. Cerebral dopamine depletion is the hallmark of Parkinson's disease.[26] Other pathological states have also been associated with dopamine dysfunction, such as schizophrenia, autism, and attention deficit hyperactivity disorder, as well as drug abuse.
Dopamine is closely associated with reward-seeking behaviors, such as approach, consumption, and addiction.[26] Recent researches suggest that the firing of dopaminergic neurons is a motivational substance as a consequence of reward-anticipation. This hypothesis is based on the evidence that, when a reward is greater than expected, the firing of certain dopaminergic neurons increases, which consequently increases desire or motivation towards the reward.[26] However, recent research finds that while some dopaminergic neurons react in the way expected of reward neurons, others do not and seem to respond in regard to unpredictability.[27] This research finds the reward neurons predominate in the ventromedial region in the substantia nigra pars compacta as well as the ventral tegmental area. Neurons in these areas project mainly to the ventral striatum and thus might transmit value-related information in regard to reward values.[27] The nonreward neurons are predominate in the dorsolateral area of the substantia nigra pars compacta which projects to the dorsal striatum and may relate to orienting behaviour.[27] It has been suggested that the difference between these two types of dopaminergic neurons arises from their input: reward-linked ones have input from the basal forebrain while the nonreward-related ones from the lateral habenula.[27]
Animal studies
Clues to dopamine's role in motivation, desire, and pleasure have come from studies performed on animals. In one such study, rats were depleted of dopamine by up to 99 percent in the nucleus accumbens and neostriatum using 6-hydroxydopamine.[26] With this large reduction in dopamine, the rats would no longer eat by their own volition. The researchers then force-fed the rats food and noted whether they had the proper facial expressions indicating whether they liked or disliked it. The researchers of this study concluded that the reduction in dopamine did not reduce the rat's consummatory pleasure, only the desire to actually eat. In another study, mutant hyperdopaminergic (increased dopamine) mice show higher "wanting" but not "liking" of sweet rewards.[28]
The effects of drugs that reduce dopamine activity
In humans, drugs that reduce dopamine activity (neuroleptics, e.g. antipsychotics) have been shown to reduce motivation, cause anhedonia (inability to experience pleasure), and long-term use has been associated with the irreversible movement disorder, tardive dyskinesia.[29] Furthermore, antipsychotic drugs are associated with weight gain, diabetes, lactation, gynecomastia, drooling, dysphoria, fatigue, sexual dysfunction, and heart rhythm problems. Selective D2/D3 agonists pramipexole and ropinirole, used to treat restless legs syndrome (RLS), have limited anti-anhedonic properties as measured by the Snaith-Hamilton Pleasure Scale (SHAPS).[30]
Opioid and cannabinoid transmission
Opioid and cannabinoid transmission instead of dopamine may modulate consummatory pleasure and food palatability (liking).[31] This could explain why animals' "liking" of food is independent of brain dopamine concentration. Other consummatory pleasures, however, may be more associated with dopamine. One study found that both anticipatory and consummatory measures of sexual behavior (male rats) were disrupted by DA receptor antagonists.[32] Libido can be increased by drugs that affect dopamine, but not by drugs that affect opioid peptides or other neurotransmitters.
Sociability
Sociability is also closely tied to dopamine neurotransmission. Low D2 receptor-binding is found in people with social anxiety. Traits common to negative schizophrenia (social withdrawal, apathy, anhedonia) are thought to be related to a hypodopaminergic state in certain areas of the brain. In instances of bipolar disorder, manic subjects can become hypersocial, as well as hypersexual.[citation needed] This is credited to an increase in dopamine, because mania can be reduced by dopamine-blocking anti-psychotics.[33]
Processing of pain
Dopamine has been demonstrated to play a role in pain processing in multiple levels of the central nervous system including the spinal cord,[34] periaqueductal gray (PAG),[35] thalamus,[36] basal ganglia,[37][38] insular cortex,[39][40] and cingulate cortex.[41] Accordingly, decreased levels of dopamine have been associated with painful symptoms that frequently occur in Parkinson's disease.[42] Abnormalities in dopaminergic neurotransmission have also been demonstrated in painful clinical conditions, including burning mouth syndrome,[43] fibromyalgia,[44][45] and restless legs syndrome.[46] In general, the analgesic capacity of dopamine occurs as a result of dopamine D2 receptor activation; however, exceptions to this exist in the PAG, in which dopamine D1 receptor activation attenuates pain presumably via activation of neurons involved in descending inhibition.[47] In addition, D1 receptor activation in the insular cortex appears to attenuate subsequent pain-related behavior.
Salience
Dopamine may also have a role in the salience of potentially important stimuli, such as sources of reward or of danger.[48] This hypothesis argues that dopamine assists decision-making by influencing the priority, or level of desire, of such stimuli to the person concerned.
Behavior disorders
Deficient dopamine neurotransmission is implicated in attention-deficit hyperactivity disorder, and stimulant medications used to successfully treat the disorder increase dopamine neurotransmission, leading to decreased symptoms.[49] Consistent with this hypothesis, dopaminergic pathways have a role in inhibitory action control and the inhibition of the tendency to make unwanted actions.[50]
The long term use of levodopa in Parkinson's disease has been linked to dopamine dysregulation syndrome.[51]
Latent inhibition and creative drive
Dopamine in the mesolimbic pathway increases general arousal and goal directed behaviors and decreases latent inhibition; all three effects increase the creative drive of idea generation. This has led to a three-factor model of creativity involving the frontal lobes, the temporal lobes, and mesolimbic dopamine.[52]
Chemoreceptor trigger zone
Dopamine is one of the neurotransmitters implicated in the control of nausea and vomiting via interactions in the chemoreceptor trigger zone. Metoclopramide is a D2-receptor antagonist that functions as a prokinetic/antiemetic.
Dopaminergic mind hypothesis
The dopaminergic mind hypothesis seeks to explain the differences between modern humans and their hominid relatives by focusing on changes in dopamine.[53] It theorizes that increased levels of dopamine were part of a general physiological adaptation due to an increased consumption of meat around two million years ago in Homo habilis, and later enhanced by changes in diet and other environmental and social factors beginning approximately 80,000 years ago. Under this theory, the "high-dopamine" personality is characterized by high intelligence, a sense of personal destiny, a religious/cosmic preoccupation, an obsession with achieving goals and conquests, an emotional detachment that in many cases leads to ruthlessness, and a risk-taking mentality. High levels of dopamine are proposed to underlie increased psychological disorders in industrialized societies. According to this hypothesis, a "dopaminergic society" is an extremely goal-oriented, fast-paced, and even manic society, "given that dopamine is known to increase activity levels, speed up our internal clocks and create a preference for novel over unchanging environments."[53] In the same way that high-dopamine individuals lack empathy and exhibit a more masculine behavioral style, dopaminergic societies are "typified by more conquest, competition, and aggression than nurturance and communality."[53] Although behavioral evidence and some indirect anatomical evidence (e.g., enlargement of the dopamine-rich striatum in humans)[54] support a dopaminergic expansion in humans, there is still no direct evidence that dopamine levels are markedly higher in humans relative to other apes.[55] However, recent discoveries about the sea-side settlements of early man may provide evidence of dietary changes consistent with this hypothesis.[56]
Links to psychosis
Abnormally high dopaminergic transmission has been linked to psychosis and schizophrenia.[57] Increased dopaminergic functional activity, specifically in the mesolimbic pathway, is found in schizophrenic individuals. Anti-psychotic medications act largely as dopamine antagonists, inhibiting dopamine at the receptor level, and thereby blocking the effects of the neurochemical in a dose-dependant manner. The older, so-called typical antipsychotics most commonly act on D2 receptors,[58] while the atypical drugs also act on D1, D3 and D4 receptors.[59][60] The finding that drugs such as amphetamines, methamphetamine and cocaine, which can increase dopamine levels by more than tenfold,[61] can temporarily cause psychosis, provides further evidence for this link.[62]
Therapeutic use
Levodopa is a dopamine precursor used in various forms to treat Parkinson's disease and dopa-responsive dystonia. It is typically co-administered with an inhibitor of peripheral decarboxylation (DDC, dopa decarboxylase), such as carbidopa or benserazide. Inhibitors of alternative metabolic route for dopamine by catechol-O-methyl transferase are also used. These include entacapone and tolcapone.
Nonneural functions
Immunoregulatory
Dopamine acts upon receptors present on immune cells, with all subtypes of dopamine receptors found on leukocytes. There is low expression of receptors on T lymphocytes and monocytes, moderate expression on neutrophils and eosinophils, and high expression on B cells and natural killer cells.[63] The sympathetic innervation of lymphoid tissues is dopaminergic, and increases during stress.[64] Dopamine can also affect immune cells in the spleen, bone marrow, and blood circulation.[65] In addition, dopamine can be synthesized and released by the immune cells themselves.[66][67]
The effects of dopamine on immune cells depend upon their physiological state. While dopamine activates resting T cells, it inhibits them when they are activated. Disorders such as schizophrenia and Parkinson's disease, in which there are changes in brain dopamine receptors and dopamine signaling pathways, are also associated with altered immune functioning.[68]
Peripheral effects
Dopamine also has effects when administered through an IV line outside the central nervous system. The brand name of this preparation is known as Intropin. The effects in this form are dose dependent.
- Dosages from 2 to 5 μg/kg/min are considered the "renal dose."[69] At this low dosage, dopamine binds D1 receptors, dilating blood vessels, increasing blood flow to renal, mesenteric, and coronary arteries; and increasing overall renal perfusion.[70] Dopamine therefore has a diuretic effect, potentially increasing urine output from 5 ml/kg/hr to 10 ml/kg/hr.[citation needed]
- Intermediate dosages from 5 to 10 μg/kg/min additionally have a positive inotropic and chronotropic effect through increased β1 receptor activation. It is used in patients with shock or heart failure to increase cardiac output and blood pressure.[70] Dopamine begins to affect the heart at the lower doses, from about 3 μg/kg/min IV.[71]
- High doses from 10 to 20 μg/kg/min is the "pressor" dose.[citation needed] This dose causes vasoconstriction, increases systemic vascular resistance, and increases blood pressure through α1 receptor activation;[70] but can cause the vessels in the kidneys to constrict to the point where they will become non-functional.[citation needed]
Renal effects
Dopamine induces natriuresis (sodium loss) in the kidneys.[72][73]
Dopamine and fruit browning
Polyphenol oxidases (PPOs) are a family of enzymes responsible for the browning of fresh fruits and vegetables when they are cut or bruised. These enzymes use molecular oxygen (O2) to oxidise various 1,2-diphenols to their corresponding quinones. The natural substrate for PPOs in bananas is dopamine. The product of their oxidation, dopamine quinone, spontaneously oxidises to other quinones. The quinones then polymerise and condense with amino acids and proteins to form brown pigments known as melanins. The quinones and melanins derived from dopamine may help protect damaged fruit and vegetables against growth of bacteria and fungi.[74]
See also
- Addiction
- Amphetamine
- Antipsychotic
- Catecholamine
- Catechol-O-methyl transferase
- Classical conditioning
- Operant conditioning
- Cocaine
- Depression
- Dopamine hypothesis of schizophrenia
- Dopamine reuptake inhibitors
- Dopaminergic
- Methylphenidate
- Neurotransmitter
- Parkinson's disease
- Prolactinoma
- Schizophrenia
- Selegiline
- Serotonin
References
- ↑ http://www.encyclopedia.com/doc/1O87-ventraltegmentalarea.html Reference for VTA.
- ↑ Fahn, Stanley, "The History of Levodopa as it Pertains to Parkinson's disease," Movement Disorder Society’s 10th International Congress of Parkinson's Disease and Movement Disorders on November 1, 2006, in Kyoto, Japan.
- ↑ Benes, F.M. Carlsson and the discovery of dopamine. Trends in Pharmacological Sciences, Volume 22, Issue 1, 1 January 2001, Pages 46-47.
- ↑ [1]
- ↑ [2]
- ↑ Peter Redgrave, Kevin Gurney (2006). "The short-latency dopamine signal: a role in discovering novel actions?". Nature Reviews Neuroscience. 7 (12): 967–975. doi:10.1038/nrn2022. PMID 17115078.
- ↑ Barron AB, Maleszka R, Vander Meer RK, Robinson GE (2007). "Octopamine modulates honey bee dance behavior". Proc. Natl. Acad. Sci. U.S.A. 104 (5): 1703–7. doi:10.1073/pnas.0610506104. PMC 1779631. PMID 17237217.
- ↑ Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M (2003). "Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila". J. Neurosci. 23 (33): 10495–502. PMID 14627633. Unknown parameter
|month=ignored (help) - ↑ Selcho M, Pauls D, Han KA, Stocker RF, Thum AS (2009). "The role of dopamine in Drosophila larval classical olfactory conditioning". PLoS ONE. 4 (6): e5897. doi:10.1371/journal.pone.0005897. PMC 2690826. PMID 19521527.
- ↑ Schultz, Cambridge university, UK
- ↑ Bjorklund A, Dunnett SB (2007). "Dopamine neuron systems in the brain: an update". Trends in Neurosciences. 20 (5): 194–202. doi:10.1016/j.tins.2007.03.006. PMID 17408759.
- ↑ Grace AA, (1991). "Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: A hypothesis for the eitiology of schizophrenia" (pdf). Neuroscience. 41 (1): 1–24. doi:10.1016/0306-4522(91)90196-U. PMID 1676137.
- ↑ Grace AA, Bunney BS (1984). "The control of firing pattern in nigral dopamine neurons: single spike firing" (pdf). Journal of Neuroscience. 4 (11): 2866–2876. PMID 6150070.
- ↑ Grace AA, Bunney BS (1984). "The control of firing pattern in nigral dopamine neurons: burst firing" (pdf). Journal of Neuroscience. 4 (11): 28677–2890. PMID 6150071.
- ↑ Gonon FG (1988). "Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry" (pdf). Neuroscience. 24 (1): 19–28. doi:10.1016/0306-4522(88)90307-7. PMID 3368048.
- ↑ Heijtz RD, Kolb B, Forssberg H (2007). "Motor inhibitory role of dopamine D1 receptors: implications for ADHD" (PDF). Physiol Behav. 92 (1–2): 155–160. doi:10.1016/j.physbeh.2007.05.024. PMID 17585966.
- ↑ Browman KE, Curzon P, Pan JB, Molesky AL, Komater VA, Decker MW, Brioni JD, Moreland RB, Fox GB. A-412997, a selective dopamine D4 agonist, improves cognitive performance in rats. Pharmacology, Biochemistry and Behaviour. 2005 Sep;82(1):148-55. PMID 16154186
- ↑ Ben-Jonathan N, Hnasko R (2001). "Dopamine as a Prolactin (PRL) Inhibitor" (PDF). Endocrine Reviews. 22 (6): 724–763. doi:10.1210/er.22.6.724. PMID 11739329.
- ↑ Dopamine Involved In Aggression - Medical News Today
- ↑ Vanderbilt University (2008, January 15). Aggression As Rewarding As Sex, Food And Drugs, New Research Shows. ScienceDaily.
- ↑ Giuliano, F. (2001). "Dopamine and male sexual function". Eur Urol. 40 (6): 601–608. doi:10.1159/000049844. PMID 11805404. Unknown parameter
|coauthors=ignored (help) - ↑ Giuliano, F. (2001). "Dopamine and sexual function". Int J Impot Res. 13 (Suppl 3): S18–S28. doi:10.1038/sj.ijir.3900719. PMID 11477488. Unknown parameter
|coauthors=ignored (help) - ↑ Yadid G, Pacak K, Golomb E, Harvey-White JD, Lieberman DM, Kopin IJ, Goldstein DS (1993). "Glycine stimulates striatal dopamine release in conscious rats". British journal of pharmacology. 110 (1): 50–3. PMID 8220914.
- ↑ Ericson M, Molander A, Stomberg R, Söderpalm B (2006). "Taurine elevates dopamine levels in the rat nucleus accumbens; antagonism by strychnine". The European journal of neuroscience. 23 (11): 3225–9. PMID 16820013.
- ↑ Ruotsalainen M, Heikkilä M, Lillsunde P, Seppälä T, Ahtee L (1996). "Taurine infused intrastriatally elevates, but intranigrally decreases striatal extracellular dopamine concentration in anaesthetised rats". Journal of Neural Transmission. 103 (8): 935–46. doi:10.1007/BF01291784.
- ↑ 26.0 26.1 26.2 26.3 26.4 Arias-Carrión O, Pöppel E (2007). "Dopamine, learning and reward-seeking behavior". Act Neurobiol Exp. 67 (4): 481–488.
- ↑ 27.0 27.1 27.2 27.3 Matsumoto M, Hikosaka O. (2009). Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 459: 837-41. PMID 19448610 doi:10.1038/nature08028
- ↑ Peciña S, Cagniard B, Berridge K, Aldridge J, Zhuang X (2003). "Hyperdopaminergic mutant mice have higher "wanting" but not "liking" for sweet rewards". J Neurosci. 23 (28): 9395–402. PMID 14561867.
- ↑ Lambert M, Schimmelmann B, Karow A, Naber D (2003). "Subjective well-being and initial dysphoric reaction under antipsychotic drugs - concepts, measurement and clinical relevance". Pharmacopsychiatry. 36 (Suppl 3): S181–90. doi:10.1055/s-2003-45128. PMID 14677077.
- ↑ Lemke M, Brecht H, Koester J, Kraus P, Reichmann H (2005). "Anhedonia, depression, and motor functioning in Parkinson's disease during treatment with pramipexole". J Neuropsychiatry Clin Neurosci. 17 (2): 214–20. doi:10.1176/appi.neuropsych.17.2.214. PMID 15939976.
- ↑ Peciña S, Berridge K (2005). "Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness?". J Neurosci. 25 (50): 11777–86. doi:10.1523/JNEUROSCI.2329-05.2005. PMID 16354936.
- ↑ Pfaus J, Phillips A (1991). "Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat". Behav Neurosci. 105 (5): 727–43. doi:10.1037/0735-7044.105.5.727. PMID 1840012.
- ↑ http://bipolar.about.com/cs/menu_treat/a/0312_treatmania_3.htm
- ↑ Jensen TS, Yaksh TL.Effects of an intrathecal dopamine agonist, apomorphine, on thermal and chemical evoked noxious responses in rats.Brain Res. 1984 Apr 2;296(2):285-93
- ↑ Flores JA, El Banoua F, Galán-Rodríguez B, Fernandez-Espejo E.Opiate anti-nociception is attenuated following lesion of large dopamine neurons of the periaqueductal grey: critical role for D1 (not D2) dopamine receptors.Pain. 2004 Jul;110(1-2):205-14.
- ↑ Shyu BC, Kiritsy-Roy JA, Morrow TJ, Casey KL.Neurophysiological, pharmacological and behavioral evidence for medial thalamic mediation of cocaine-induced dopaminergic analgesia.Brain Res. 1992 Feb 14;572(1-2):216-23.
- ↑ Chudler EH, Dong WK.The role of the basal ganglia in nociception and pain.Pain. 1995 Jan;60(1):3-38
- ↑ Altier N, Stewart J.The role of dopamine in the nucleus accumbens in analgesia.Life Sci. 1999;65(22):2269-87
- ↑ Burkey AR, Carstens E, Jasmin L.Dopamine reuptake inhibition in the rostral agranular insular cortex produces antinociception.J Neurosci. 1999 May 15;19(10):4169-79.
- ↑ Coffeen U, López-Avila A, Ortega-Legaspi JM, del Angel R, López-Muñoz FJ, Pellicer F.Dopamine receptors in the anterior insular cortex modulate long-term nociception in the rat.Eur J Pain. 2008 Jul;12(5):535-43.
- ↑ López-Avila A, Coffeen U, Ortega-Legaspi JM, del Angel R, Pellicer F.Dopamine and NMDA systems modulate long-term nociception in the rat anterior cingulate cortex.Pain. 2004 Sep;111(1-2):136-43.
- ↑ Brefel-Courbon C, Payoux P, Thalamas C, Ory F, Quelven I, Chollet F, Montastruc JL, Rascol O.Effect of levodopa on pain threshold in Parkinson's disease: a clinical and positron emission tomography study.Mov Disord. 2005 Dec;20(12):1557-63.
- ↑ Jääskeläinen SK, Rinne JO, Forssell H, Tenovuo O, Kaasinen V, Sonninen P, Bergman J.Role of the dopaminergic system in chronic pain -- a fluorodopa-PET study.Pain. 2001 Feb 15;90(3):257-60.
- ↑ Wood PB, Patterson JC 2nd, Sunderland JJ, Tainter KH, Glabus MF, Lilien DL.Reduced presynaptic dopamine activity in fibromyalgia syndrome demonstrated with positron emission tomography: a pilot study.J Pain. 2007 Jan;8(1):51-8.
- ↑ Wood PB, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner EA, Bushnell MC, Chizh BA.Fibromyalgia patients show an abnormal dopamine response to pain.Eur J Neurosci. 2007 Jun;25(12):3576-82.
- ↑ Cervenka S, Pålhagen SE, Comley RA, Panagiotidis G, Cselényi Z, Matthews JC, Lai RY, Halldin C, Farde L.Support for dopaminergic hypoactivity in restless legs syndrome: a PET study on D2-receptor binding.Brain. 2006 Aug;129(Pt 8):2017-28.
- ↑ Wood PB.Role of central dopamine in pain and analgesia.Expert Rev Neurother. 2008 May;8(5):781-97.
- ↑ Schultz W (2002). "Getting formal with dopamine and reward". Neuron. 36 (2): 241–263. doi:10.1016/S0896-6273(02)00967-4. PMID 12383780.
- ↑ "A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes". Cambridge Journals. Retrieved 2009-04-20.
- ↑ Colzato LS, van den Wildenberg WP, van Wouwe NC, Pannebakker MM, Hommel B. (2009). Dopamine and inhibitory action control: evidence from spontaneous eye blink rates. Exp Brain Res. 196(3):467-74. PMID 19484465 doi:10.1007/s00221-009-1862-x
- ↑ Merims D, Giladi N (2008). "Dopamine dysregulation syndrome, addiction and behavioral changes in Parkinson's disease". Parkinsonism Relat. Disord. 14 (4): 273–80. doi:10.1016/j.parkreldis.2007.09.007. PMID 17988927.
- ↑ Flaherty, A.W, (2005). "Frontotemporal and dopaminergic control of idea generation and creative drive". Journal of Comparative Neurology. 493 (1): 147–153. doi:10.1002/cne.20768. PMC 2571074. PMID 16254989.
- ↑ 53.0 53.1 53.2 Previc F (2009). The Dopaminergic Mind in Human Evolution and History Cambridge University Press. ISBN 978-0-521-51699-0.
- ↑ Rapoport, S. I. (1990). Integrated phylogeny of the primate brain, with special reference to humans and their diseases. Brain Research Reviews, 15, 267-294.
- ↑ Raghanti, M. A., Stimpson, C. D., Marcinkiewicz, J. L., Erwin, J. M., Hof, P. R., & Sherwood, C. C. (2008a). Cortical dopaminergic innervation among humans, chimpanzees, and macaque monkeys: A comparative study. Neuroscience, 155, 203-20
- ↑ http://www.scientificamerican.com/article.cfm?id=interactive-seas-saved-humanity
- ↑ "Disruption of gene interaction linked to schizophrenia". St. Jude Children's Research Hospital. Retrieved 2006-07-06.
- ↑ http://www.williams.edu/imput/synapse/pages/IIIB5.htm
- ↑ http://bjp.rcpsych.org/cgi/content/full/181/4/271
- ↑ http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T99-3YYTH6C-3P&_user=10&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=7fda10d8dad0b9937580f6371eebb2d5
- ↑ Methamphetamine 101
- ↑ Lieberman, J.A. (1997). "Provocative tests with psychostimulant drugs in schizophrenia". Psychopharmacology (Berl). 91 (4): 415–433. doi:10.1007/BF00216006. PMID 2884687. Unknown parameter
|coauthors=ignored (help);|access-date=requires|url=(help) - ↑ McKenna F, McLaughlin PJ, Lewis BJ, Sibbring GC, Cummerson JA, Bowen-Jones D, Moots RJ. (2002). Dopamine receptor expression on human T- and B-lymphocytes, monocytes, neutrophils, eosinophils and NK cells: a flow cytometric study. J Neuroimmunol. 132(1-2):34-40. PMID 12417431
- ↑ Mignini F, Streccioni V, Amenta F. (2003). Autonomic innervation of immune organs and neuroimmune modulation. Auton Autacoid Pharmacol. 23(1):1-25. PMID 14565534
- ↑ Basu S, Dasgupta PS. (2000). Dopamine, a neurotransmitter, influences the immune system. J Neuroimmunol. 102(2):113-24. PMID 10636479
- ↑ Bergquist J, Tarkowski A, Ekman R, Ewing A. (1994). Discovery of endogenous catecholamines in lymphocytes and evidence for catecholamine regulation of lymphocyte function via an autocrine loop. Proc Natl Acad Sci U S A. 91(26):12912-6. PMID 7809145
- ↑ Cosentino M, Fietta AM, Ferrari M, Rasini E, Bombelli R, Carcano E, Saporiti F, Meloni F, Marino F, Lecchini S. (2007). Human CD4+CD25+ regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood. 109(2):632-42. PMID 16985181
- ↑ Sarkar C, Basu B, Chakroborty D, Dasgupta PS, Basu S. (2010). The immunoregulatory role of dopamine: an update. Brain Behav Immun. 24(4):525-8. doi:10.1016/j.bbi.2009.10.015 PMID 19896530
- ↑ "Renal Vasodilatory Action of Dopamine in Patients With Heart Failure: Magnitude of Effect and Site of Action". Circulation. 2008;117:200-205. Retrieved 2009-04-20.
- ↑ 70.0 70.1 70.2 Shen, Howard (2008). Illustrated Pharmacology Memory Cards: PharMnemonics. Minireview. p. 8. ISBN 1-59541-101-1.
- ↑ "Dopamine and Dextrose". Drugs.com. Retrieved 2009-04-20.
- ↑ [3]
- ↑ [4]
- ↑ Mayer, AM (2006). "Polyphenol oxidases in plants and fungi: Going places? A review". Phytochemistry. 67 (21): 2318–2331. doi:10.1016/j.phytochem.2006.08.006. PMID 16973188.
External links
| File:Wiktionary-logo-en-v2.svg | Look up Dopamine in Wiktionary, the free dictionary. |
Template:Phenethylamines Template:Cardiac stimulants excluding cardiac glycosides
- Pages with script errors
- Pages with reference errors
- CS1 maint: Multiple names: authors list
- Pages with citations using unsupported parameters
- Pages using citations with accessdate and no URL
- Chemical articles with multiple compound IDs
- Multiple chemicals in an infobox that need indexing
- Chemical articles with multiple CAS registry numbers
- Articles without EBI source
- Articles without KEGG source
- ECHA InfoCard ID from Wikidata
- Chembox having DSD data
- Chemical articles with unknown parameter in Chembox
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Pages with broken file links
- All articles with unsourced statements
- Articles with unsourced statements from April 2010
- Articles with invalid date parameter in template
- Articles with unsourced statements from May 2010
- Articles with unsourced statements from August 2008
- Catecholamines
- Hormones of the hypothalamus
- Inotropic agents
- Motivation
- Neurotransmitters