Dapsone (topical)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Dapsone (topical) is an anti-infective agent that is FDA approved for the treatment of acne vulgaris. Common adverse reactions include nausea, vomiting, abdominal pain, pancreatitis, vertigo, blurred vision, and tinnitus.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Dapsone® Gel, 5%, is indicated for the topical treatment of acne vulgaris.
Dosing Information
- For topical use only. Not for oral, ophthalmic, or intravaginal use.
- After the skin is gently washed and patted dry, apply approximately a pea-sized amount of Dapsone®Gel, 5%, in a thin layer to the acne affected areas twice daily. Rub in Dapsone ® Gel, 5%, gently and completely. Dapsone® Gel, 5%, is gritty with visible drug substance particles. Wash hands after application of Dapsone® Gel, 5%.
- If there is no improvement after 12 weeks, treatment with Dapsone ® Gel, 5%, should be reassessed.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dapsone (topical) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dapsone (topical) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Dapsone (topical) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dapsone (topical) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dapsone (topical) in pediatric patients.
Contraindications
- None.
Warnings
- Hematological Effects
- Oral dapsone treatment has produced dose-related hemolysis and hemolytic anemia. Individuals with glucose-6-phosphate dehydrogenase (G6PD) deficiency are more prone to hemolysis with the use of certain drugs. G6PD deficiency is most prevalent in populations of African, South Asian, Middle Eastern, and Mediterranean ancestry.
- There was no evidence of clinically relevant hemolysis or anemia in patients treated with Dapsone ®Gel, 5%, including patients who were G6PD deficient. Some subjects with G6PD deficiency using Dapsone ® Gel developed laboratory changes suggestive of mild hemolysis.
- If signs and symptoms suggestive of hemolytic anemia occur, Dapsone ® Gel, 5% should be discontinued. Dapsone ® Gel, 5% should not be used in patients who are taking oral dapsone or antimalarial medications because of the potential for hemolytic reactions. Combination of Dapsone ®Gel, 5%, with trimethoprim/sulfamethoxazole (TMP/SMX) may increase the likelihood of hemolysis in patients with G6PD deficiency.
- Peripheral Neuropathy
- Peripheral neuropathy (motor loss and muscle weakness) has been reported with oral dapsone treatment. No events of peripheral neuropathy were observed in clinical trials with topical Dapsone ®Gel, 5% treatment.
- Skin
- Skin reactions (toxic epidermal necrolysis, erythema multiforme, morbilliform and scarlatiniform reactions, bullous and exfoliative dermatitis, erythema nodosum, and urticaria) have been reported with oral dapsone treatment. These types of skin reactions were not observed in clinical trials with topical Dapsone ® Gel, 5% treatment.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under prescribed conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- Serious adverse reactions reported in patients treated with Dapsone ® Gel, 5%, during clinical trials included but were not limited to the following:
- Nervous system/Psychiatric – Suicide attempt, tonic clonic movements.
- Gastrointestinal – Abdominal pain, severe vomiting, pancreatitis.
- Other – Severe pharyngitis
- In the clinical trials, a total of 12 out of 4032 patients were reported to have depression (3 of 1660 treated with vehicle and 9 of 2372 treated with Dapsone ® Gel, 5%). Psychosis was reported in 2 of 2372 patients treated with Dapsone ® Gel, 5%, and in 0 of 1660 patients treated with vehicle.
- Combined contact sensitization/irritation studies with Dapsone ® Gel, 5%, in 253 healthy subjects resulted in at least 3 subjects with moderate erythema. Dapsone ® Gel, 5%, did not induce phototoxicity or photoallergy in human dermal safety studies.
- Dapsone ® Gel, 5%, was evaluated for 12 weeks in four controlled studies for local cutaneous events in 1819 patients. The most common events reported from these studies include oiliness/peeling, dryness, and erythema. These data are shown by severity in TABLE 1 below.
- One patient treated with Dapsone ® Gel in the clinical trials had facial swelling which led to discontinuation of medication.
- In addition, 486 patients were evaluated in a 12 month safety study. The adverse event profile in this study was consistent with that observed in the vehicle-controlled studies.
Experience with Oral Use of Dapsone
- Although not observed in the clinical trials with Dapsone ® Gel (topical dapsone) serious adverse reactions have been reported with oral use of dapsone, including agranulocytosis, hemolytic anemia, peripheral neuropathy (motor loss and muscle weakness), and skin reactions (toxic epidermal necrolysis, erythema multiforme, morbilliform and scarlatiniform reactions, bullous and exfoliative dermatitis, erythema nodosum, and urticaria).
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Dapsone (topical) in the drug label.
Drug Interactions
- Trimethoprim-Sulfamethoxazole
- A drug-drug interaction study evaluated the effect of the use of Dapsone ® Gel, 5%, in combination with double strength (160 mg/800 mg) trimethoprim-sulfamethoxazole (TMP/SMX). During co-administration, systemic levels of TMP and SMX were essentially unchanged. However, levels of dapsone and its metabolites increased in the presence of TMP/SMX. Systemic exposure (AUC0-12) of dapsone and N-acetyl-dapsone (NAD) were increased by about 40% and 20% respectively in the presence of TMP/SMX. Notably, systemic exposure (AUC0-12) of dapsone hydroxylamine (DHA) was more than doubled in the presence of TMP/SMX. Exposure from the proposed topical dose is about 1% of that from the 100 mg oral dose, even when co-administered with TMP/SMX.
- Topical Benzoyl Peroxide
- Topical application of Dapsone ® Gel followed by benzoyl peroxide in subjects with acne vulgaris resulted in a temporary local yellow or orange discoloration of the skin and facial hair (reported by 7 out of 95 subjects in a clinical study) with resolution in 4 to 57 days.
- Drug Interactions with Oral Dapsone
- Certain concomitant medications (such as rifampin, anticonvulsants, St. John's wort) may increase the formation of dapsone hydroxylamine, a metabolite of dapsone associated with hemolysis. With oral dapsone treatment, folic acid antagonists such as pyrimethamine have been noted to possibly increase the likelihood of hematologic reactions.
Use in Specific Populations
Pregnancy
- Teratogenic Effects: Pregnancy Category C
- There are no adequate and well controlled studies in pregnant women. Dapsone has been shown to have an embryocidal effect in rats and rabbits when administered orally in doses of 75 mg/kg/day and 150 mg/kg/day (approximately 800 and 500 times the systemic exposure observed in human females as a result of use of the maximum recommended topical dose, based on AUC comparisons), respectively. These effects were probably secondary to maternal toxicity. Dapsone ® Gel, 5%, should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Dapsone (topical) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Dapsone (topical) during labor and delivery.
Nursing Mothers
- Although systemic absorption of dapsone following topical application of Dapsone ® Gel, 5%, is minimal relative to oral dapsone administration, it is known that dapsone is excreted in human milk. Because of the potential for oral dapsone to cause adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue Dapsone ® Gel, 5%, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and efficacy was evaluated in 1169 children aged 12-17 years old treated with Dapsone ® Gel, 5%, in the clinical studies. The adverse event rate for Dapsone ® Gel, 5%, was similar to the vehicle control group. Safety and efficacy was not studied in pediatric patients less than 12 years of age, therefore Dapsone ® Gel, 5%, is not recommended for use in this age group.
Geriatic Use
- Clinical studies of Dapsone ® Gel, 5%, did not include sufficient number of patients aged 65 and over to determine whether they respond differently from younger patients.
Gender
There is no FDA guidance on the use of Dapsone (topical) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Dapsone (topical) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Dapsone (topical) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Dapsone (topical) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Dapsone (topical) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Dapsone (topical) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Topical
Monitoring
There is limited information regarding Monitoring of Dapsone (topical) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Dapsone (topical) in the drug label.
Overdosage
- Dapsone ® Gel, 5%, is not for oral use. If oral ingestion occurs, medical advice should be sought.
Pharmacology

Mechanism of Action
- The mechanism of action of dapsone gel in treating acne vulgaris is not known.
Microbiology
- In Vivo Activity: No microbiology or immunology studies were conducted during dapsone gel clinical trials.
- Drug Resistance: No dapsone resistance studies were conducted during dapsone gel clinical trials. Because no microbiology studies were done, there are no data available as to whether dapsone treatment may have resulted in decreased susceptibility of Propionibacterium acnes, an organism associated with acne, to other antimicrobials that may be used to treat acne. Therapeutic resistance to dapsone has been reported for Mycobacterium leprae, when patients have been treated with oral dapsone.
Structure
- Dapsone ® Gel, 5%, contains dapsone, a sulfone, in an aqueous gel base for topical dermatologic use.
- Dapsone ® Gel, 5% is a gritty translucent material with visible drug substance particles. Chemically, dapsone has an empirical formula of C12H12N2O2S. It is a white, odorless crystalline powder that has a molecular weight of 248. Dapsone's chemical name is 4,4'-diaminodiphenylsulfone and its structural formula is:
- Each gram of Dapsone ® Gel, 5%, contains 50 mg of dapsone, USP, in a gel of carbomer homopolymer type C; diethylene glycol monoethyl ether, NF; methylparaben, NF; sodium hydroxide, NF; and purified water, USP.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Dapsone (topical) in the drug label.
Pharmacokinetics
- An open-label study compared the pharmacokinetics of dapsone after Dapsone ® Gel, 5%, (110 ± 60 mg/day) was applied twice daily (~BSA 22.5%) for 14 days (n=18) with a single 100 mg dose of oral dapsone administered to a subgroup of patients (n=10) in a crossover design. On Day 14 the mean dapsone AUC0-24h was 415 ± 224 ng•h/mL for Dapsone ® Gel, 5%, whereas following a single 100 mg dose of oral dapsone the AUC0-infinity was 52,641 ± 36,223 ng•h/mL. Exposure after the oral dose of 100 mg dapsone was approximately 100 times greater than after the topical Dapsone ® Gel, 5% dose, twice a day.
- In a long-term safety study of Dapsone ® Gel, 5% treatment, periodic blood samples were collected up to 12 months to determine systemic exposure of dapsone and its metabolites in approximately 500 patients. Based on the measurable dapsone concentrations from 408 patients (M=192, F=216), obtained at month 3, neither gender, nor race appeared to affect the pharmacokinetics of dapsone. Similarly, dapsone exposures were approximately the same between the age groups of 12-15 years (N=155) and those greater than or equal to 16 years (N=253). There was no evidence of increasing systemic exposure to dapsone over the study year in these patients.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Dapsone was not mutagenic in a bacterial reverse mutation assay (Ames test) using S. typhimurium and E. coli, with and without metabolic activation and was negative in a micronucleus assay conducted in mice. Dapsone increased both numerical and structural aberrations in a chromosome aberration assay conducted with Chinese hamster ovary (CHO) cells.
- Dapsone was not carcinogenic to rats when orally administered to females for 92 weeks or males for 100 weeks at dose levels up to 15 mg/kg/day (approximately 160 times the systemic exposure observed in human males and 300 times the systemic exposure observed in human females as a result of use of the maximum recommended topical dose, based on AUC comparisons).
- No evidence of potential to induce carcinogenicity was obtained in a dermal study in which dapsone gel was topically applied to Tg. AC transgenic mice for approximately 26 weeks. Dapsone concentrations of 3%, 5%, and 10% were evaluated; 3% material was judged to be the maximum tolerated dosage.
- Dapsone ® Gel, 5%, did not increase the rate of formation of ultraviolet light-induced skin tumors when topically applied to hairless mice in a 12-month photo carcinogenicity study.
- The effects of dapsone on fertility and general reproduction performance were assessed in male and female rats following oral (gavage) dosing. Dapsone reduced sperm motility at dosages of 3 mg/kg/day or greater (approximately 17 times the systemic exposure observed in human males as a result of use of the maximum recommended topical dose, based on AUC comparisons). The mean numbers of embryo implantations and viable embryos were significantly reduced in untreated females mated with males that had been dosed at 12 mg/kg/day or greater (approximately 70 times the systemic exposure observed in human males as a result of use of the maximum recommended topical dose, based on AUC comparisons), presumably due to reduced numbers or effectiveness of sperm, indicating impairment of fertility. Dapsone had no effect on male fertility at dosages of 2 mg/kg/day or less (approximately 13 times the systemic exposure observed in human males as a result of use of the maximum recommended topical dose, based on AUC comparisons). When administered to female rats at a dosage of 75 mg/kg/day (approximately 800 times the systemic exposure observed in human females as a result of use of the maximum recommended topical dose, based on AUC comparisons) for 15 days prior to mating and for 17 days thereafter, dapsone reduced the mean number of implantations, increased the mean early resorption rate, and reduced the mean litter size. These effects were probably secondary to maternal toxicity.
- Dapsone was assessed for effects on perinatal/postnatal pup development and postnatal maternal behavior and function in a study in which dapsone was orally administered to female rats daily beginning on the seventh day of gestation and continuing until the twenty-seventh day postpartum. Maternal toxicity (decreased body weight and food consumption) and developmental effects (increase in stillborn pups and decreased pup weight) were seen at a dapsone dose of 30 mg/kg/day (approximately 500 times the systemic exposure observed in human females as a result of use of the maximum recommended topical dose, based on AUC comparisons). No effects were observed on the viability, physical development, behavior, learning ability, or reproductive function of surviving pups.
Clinical Studies
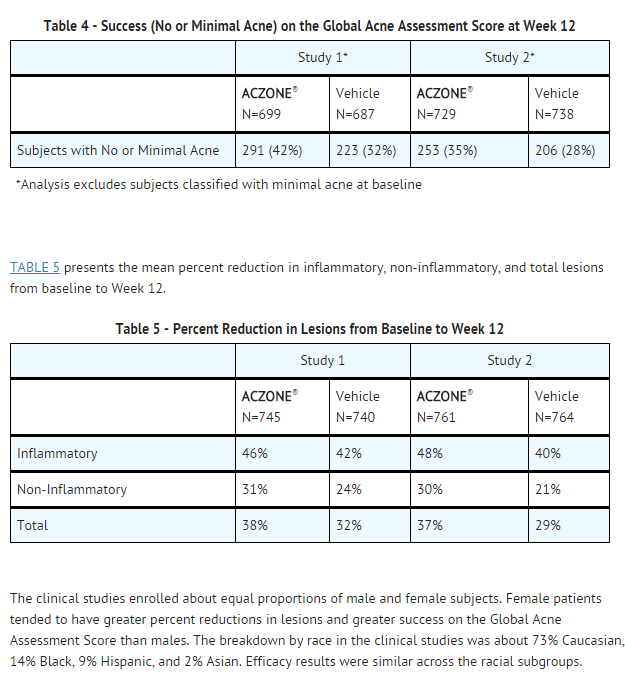
- Two randomized, double-blind, vehicle-controlled, clinical studies were conducted to evaluate Dapsone ® Gel, 5%, for the treatment of patients with acne vulgaris (N=1475 and 1525). The studies were designed to enroll patients 12 years of age and older with 20 to 50 inflammatory and 20 to 100 non-inflammatory lesions at baseline. In these studies patients applied either Dapsone® Gel, 5%, or vehicle control twice daily for up to 12 weeks. Efficacy was evaluated in terms of success on the Global Acne Assessment Score (no or minimal acne) and in the percent reduction in inflammatory, non-inflammatory, and total lesions.
- The Global Acne Assessment Score was a 5-point scale as follows:
- None: no evidence of facial acne vulgaris
- Minimal: few non-inflammatory lesions (comedones) are present; a few inflammatory lesions (papules/pustules) may be present
- Mild: several to many non-inflammatory lesions (comedones) are present; a few inflammatory lesions (papules/pustules) are present
- Moderate: many non-inflammatory (comedones) and inflammatory lesions (papules/pustules) are present; no nodulo-cystic lesions are allowed
- Severe: significant degree of inflammatory disease; papules/pustules are a predominant feature; a few nodulo-cystic lesions may be present; comedones may be present.
- The success rates on the Global Acne Assessment Score (no or minimal acne) at Week 12 are presented in TABLE 4.
- The clinical studies enrolled about equal proportions of male and female subjects. Female patients tended to have greater percent reductions in lesions and greater success on the Global Acne Assessment Score than males. The breakdown by race in the clinical studies was about 73% Caucasian, 14% Black, 9% Hispanic, and 2% Asian. Efficacy results were similar across the racial subgroups.
How Supplied
- Dapsone ® (dapsone) Gel, 5%, is supplied in the following size tubes:
- Professional Sample
- 5% NDC 0023-3670-03
- 3 gram laminate tube
- Commercially Available as:
- 5% NDC 0023-3670-30
- 30 gram laminate tube
- 5% NDC 0023-3670-60
- 60 gram laminate tube
- 5% NDC 0023-3670-90
- 90 gram laminate tube
- KEEP OUT OF THE REACH OF CHILDREN LESS THAN 12 YEARS OLD.
- Storage conditions:
- Store at controlled room temperature, 20°-25°C (68°-77°F), excursions permitted to 15°-30ºC (59°-86ºF). Protect from freezing.
Storage
There is limited information regarding Dapsone (topical) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Dapsone (topical) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Dapsone (topical) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Information for Patients
- Patients should use Dapsone ® Gel, 5%, as directed by the physician. Dapsone ® Gel, 5%, is for external topical use only. Dapsone ® Gel, 5%, is not for oral, ophthalmic or intravaginal use.
- Patients should not use this medication for any disorder other than that for which it was prescribed.
- Patients should report any signs of adverse reactions to their physician.
- Protect Dapsone ® Gel, 5%, from freezing.
- See Patient Labeling for additional information on safety, efficacy, general use, and storage of Dapsone ® Gel, 5%.
Precautions with Alcohol
- Alcohol-Dapsone (topical) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
ACZONE[1]
Look-Alike Drug Names
There is limited information regarding Dapsone (topical) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Dapsone (topical) |Label Name=Dapsone (topical)02.png
}}
{{#subobject:
|Label Page=Dapsone (topical) |Label Name=Dapsone (topical)03.png
}}