Trimethoprim: Difference between revisions
m (Robot: Automated text replacement (-{{SIB}} +, -{{EH}} +, -{{EJ}} +, -{{Editor Help}} +, -{{Editor Join}} +)) |
Gerald Chi (talk | contribs) m (Changed protection level for "Trimethoprim" ([Edit=Allow only autoconfirmed users] (expires 20:42, 22 January 2014 (UTC)) [Move=Allow only autoconfirmed users] (expires 20:42, 22 January 2014 (UTC)))) |
(No difference)
| |
Revision as of 20:42, 8 January 2014
 | |
| Clinical data | |
|---|---|
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 90–100% |
| Metabolism | hepatic |
| Elimination half-life | 8–10 hours |
| Excretion | renal 50–60% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C14H18N4O3 |
| Molar mass | 290.32 g/mol |
|
WikiDoc Resources for Trimethoprim |
|
Articles |
|---|
|
Most recent articles on Trimethoprim Most cited articles on Trimethoprim |
|
Media |
|
Powerpoint slides on Trimethoprim |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Trimethoprim at Clinical Trials.gov Clinical Trials on Trimethoprim at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Trimethoprim
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Trimethoprim Discussion groups on Trimethoprim Patient Handouts on Trimethoprim Directions to Hospitals Treating Trimethoprim Risk calculators and risk factors for Trimethoprim
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Trimethoprim |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Trimethoprim (INN) (IPA: Template:IPA) is a bacteriostatic antibiotic mainly used in the prophylaxis and treatment of urinary tract infections. It belongs to the class of chemotherapeutic agents known as dihydrofolate reductase inhibitors. Trimethoprim was formerly marketed by GlaxoWellcome under trade names including Proloprim, Monotrim and Triprim; but these trade names have been licensed to various generic pharmaceutical manufacturers. In clinical use it is often abbreviated TRI or TMP; its common laboratory abbreviation is W.
Mechanism of action
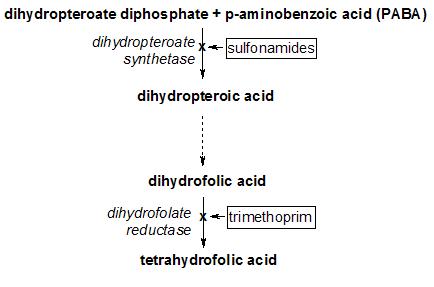
Trimethoprim acts by interfering with the action of bacterial dihydrofolate reductase, inhibiting synthesis of tetrahydrofolic acid. Tetrahydrofolic acid is an essential precursor in the de novo synthesis of the DNA nucleotide thymine. Bacteria are unable to take up folic acid from the environment (i.e. the infection host) and are thus dependent on their own de novo synthesis. Inhibition of the enzyme starves the bacteria of bases necessary for DNA replication.
Co-trimoxazole
Trimethoprim was commonly used in combination with sulfamethoxazole, a sulfonamide antibiotic, which inhibits an earlier step in the folate synthesis pathway (see diagram above). This combination, also known as co-trimoxazole, TMP-sulfa, or TMP-SMX, results in an in vitro synergistic antibacterial effect by inhibiting successive steps in folate synthesis, this claimed benefit was not seen in general clinical use.[1] [2] Its use has been declining due to reports of sulfamethoxazole bone marrow toxicity, resistance and lack greater efficacy in treating common urine and chest infections,[3][4][5][6] and side effects of antibacterial sulfonamides. As a consequence, the use of co-trimoxazole was restricted in 1995.[7]
Clinical indications
Trimethoprim, used as monotherapy, is indicated for the prophylaxis and treatment of urinary tract infections (cystitis). Co-trimoxazole, with its greater efficacy against a limited number of bacteria, remains indicated for some infections; for example, it is used as prophylaxis in patients at risk for Pneumocystis jirovecii pneumonia (e.g. AIDS patients and those with some hematological malignancies) and as therapy in Whipple's disease.
Footnotes
- ↑ Brumfitt W, Hamilton-Miller JM (1993). "Reassessment of the rationale for the combinations of sulphonamides with diaminopyrimidines". J Chemother. 5 (6): 465–9. PMID 8195839. Unknown parameter
|month=ignored (help) - ↑ Brumfitt W, Hamilton-Miller JM (1994). "Limitations of and indications for the use of co-trimoxazole". J Chemother. 6 (1): 3–11. PMID 8071675. Unknown parameter
|month=ignored (help) - ↑ Bean DC, Livermore DM, Papa I, Hall LM (2005). "Resistance among Escherichia coli to sulphonamides and other antimicrobials now little used in man". J Antimicrob Chemother. 56 (5): 962–4. PMID 16150859. Unknown parameter
|month=ignored (help) - ↑ Felmingham D, Reinert RR, Hirakata Y, Rodloff A (2002). "Increasing prevalence of antimicrobial resistance among isolates of Streptococcus pneumoniae from the PROTEKT surveillance study, and compatative in vitro activity of the ketolide, telithromycin". J Antimicrob Chemother. 50 (Suppl S1): 25–37. PMID 12239226. Unknown parameter
|month=ignored (help) - ↑ Johnson JR, Manges AR, O'Bryan TT, Riley LW (2002). "A disseminated multidrug-resistant clonal group of uropathogenic Escherichia coli in pyelonephritis". Lancet. 359 (9325): 2249–51. PMID 12103291. Unknown parameter
|month=ignored (help) - ↑ Lawrenson RA, Logie JW (2001). "Antibiotic failure in the treatment of urinary tract infections in young women". J Antimicrob Chemother. 48 (6): 895–901. PMID 11733475. Unknown parameter
|month=ignored (help) - suggest some small advantage in UTIs - ↑ "Co-trimoxazole use restricted". Drug Ther Bull. 33 (12): 92–3. 1995. PMID 8777892. Unknown parameter
|month=ignored (help)
External links
- Nucleic acid inhibitors (PDF file).
Template:Sulfonamides and trimethoprim
de:Trimethoprim hu:Trimetoprim sk:Trimetoprim sv:Trimetoprim th:ไตรเมโทพริม
- Pages with script errors
- Pages with citations using unsupported parameters
- CS1 maint: Multiple names: authors list
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Antibiotics
- Dihydrofolate reductase inhibitors