Trimethoprim
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Trimethoprim is a folic acid antagonist that is FDA approved for the treatment of uncomplicated urinary tract infection. Common adverse reactions include pruritus and rash.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Uncomplicated urinary tract infectious disease
- Dosage: 100 mg of trimethoprim every 12 hours or 200 mg of trimethoprim every 24 hours, each for 10 days.
- Caused by Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, Enterobacter species, and coagulase-negative Staphylococcus species, including S. saprophyticus.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Trimethoprim in adult patients.
Non–Guideline-Supported Use
- Bacterial infection of eye
- HIV infection - Pneumocystis pneumonia
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Trimethoprim FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Trimethoprim in pediatric patients.
Non–Guideline-Supported Use
- Bacterial infection of eye
Contraindications
Trimethoprim is contraindicated in individuals hypersensitive to trimethoprim and in those with documented megaloblastic anemia due to folate deficiency.
Warnings
- Serious hypersensitivity reactions have been reported rarely in patients on trimethoprim therapy. Trimethoprim has been reported rarely to interfere with hematopoiesis, especially when administered in large doses and/or for prolonged periods.
- The presence of clinical signs such as sore throat, fever, pallor, or purpura may be early indications of serious blood disorders.
- Complete blood counts should be obtained if any of these signs are noted in a patient receiving trimethoprim and the drug discontinued if a significant reduction in the count of any formed blood element is found.
- Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including trimethoprim tablets, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
- C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
- If CDAD is suspected or confirmed, ongoing antiobiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Adverse Reactions
Clinical Trials Experience
The adverse effects encountered most often with trimethoprim were rash and pruritus.
Dermatologic
- Rash, pruritus, and phototoxic skin eruptions. At the recommended dosage regimens of 100 mg b.i.d. or 200 mg q.d., each for 10 days, the incidence of rash is 2.9% to 6.7%. In clinical studies which employed high doses of trimethoprim, an elevated incidence of rash was noted. These rashes were maculopapular, morbilliform, pruritic, and generally mild to moderate, appearing 7 to 14 days after the initiation of therapy.
Hypersensitivity
- Rare reports of exfoliative dermatitis, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis (Lyell Syndrome), and anaphylaxis have been received.
Gastrointestinal
- Epigastric distress, nausea, vomiting, and glossitis. Elevation of serum transaminase and bilirubin has been noted, but the significance of this finding is unknown. Cholestatic jaundice has been rarely reported.
Hematologic
Metabolic
Neurologic
- Aseptic meningitis has been rarely reported.
Miscellaneous
- Fever, and increases in BUN and serum creatinine levels.
Postmarketing Experience
There is limited information regarding Trimethoprim Postmarketing Experience in the drug label.
Drug Interactions
Trimethoprim may inhibit the hepatic metabolism of phenytoin. Trimethoprim, given at a common clinical dosage, increased the phenytoin half-life by 51% and decreased the phenytoin metabolic clearance rate by 30%. When administering these drugs concurrently, one should be alert for possible excessive phenytoin effect.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C Trimethoprim has been shown to be teratogenic in the rat when given in doses 40 times the human dose. In some rabbit studies, the overall increase in fetal loss (dead and resorbed and malformed conceptuses) was associated with doses six times the human therapeutic dose.
While there are no large, well-controlled studies on the use of trimethoprim in pregnant women, Brumfitt and Pursell,3 in a retrospective study, reported the outcome of 186 pregnancies during which the mother received either placebo or trimethoprim in combination with sulfamethoxazole. The incidence of congenital abnormalities was 4.5% (3 of 66) in those who received placebo and 3.3% (4 of 120) in those receiving trimethoprim and sulfamethoxazole. There were no abnormalities in the 10 children whose mothers received the drug during the first trimester. In a separate survey, Brumfitt and Pursell also found no congenital abnormalities in 35 children whose mothers had received trimethoprim and sulfamethoxazole at the time of conception or shortly thereafter.
Because trimethoprim may interfere with folic acid metabolism, trimethoprim should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects
The oral administration of trimethoprim to rats at a dose of 70 mg/kg/day commencing with the last third of gestation and continuing through parturition and lactation caused no deleterious effects on gestation or pup growth and survival.
Pregnancy Category (AUS): B3
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Trimethoprim in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Trimethoprim during labor and delivery.
Nursing Mothers
Trimethoprim is excreted in human milk. Because trimethoprim may interfere with folic acid metabolism, caution should be exercised when trimethoprim is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 2 months have not been established. The effectiveness of trimethoprim as a single agent has not been established in pediatric patients under 12 years of age.
Geriatic Use
Clinical studies of trimethoprim tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in response between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
Case reports of hyperkalemia in elderly patients receiving trimethoprim-sulfamethoxazole have been published.6 Trimethoprim is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor potassium concentrations and to monitor renal function by calculating creatinine clearance.
Gender
There is no FDA guidance on the use of Trimethoprim with respect to specific gender populations.
Race
There is no FDA guidance on the use of Trimethoprim with respect to specific racial populations.
Renal Impairment
The use of trimethoprim in patients with a creatinine clearance of less than 15 mL/min is not recommended. For patients with a creatinine clearance of 15 to 30 mL/min, the dose should be 50 mg every 12 hours.
Hepatic Impairment
There is no FDA guidance on the use of Trimethoprim in patients with hepatic impairment.
Females of Reproductive Potential and Males
No adverse effects on fertility or general reproductive performance were observed in rats given trimethoprim in oral dosages as high as 70 mg/kg/day for males and 14 mg/kg/day for females.
Immunocompromised Patients
There is no FDA guidance one the use of Trimethoprim in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
There is limited information regarding Trimethoprim Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Trimethoprim and IV administrations.
Overdosage
Acute
Signs of acute overdosage with trimethoprim may appear following ingestion of 1 gram or more of the drug and include nausea, vomiting, dizziness, headaches, mental depression, confusion, and bone marrow depression.
Treatment consists of gastric lavage and general supportive measures. Acidification of the urine will increase renal elimination of trimethoprim. Peritoneal dialysis is not effective and hemodialysis is only moderately effective in eliminating the drug.
Chronic
Use of trimethoprim at high doses and/or for extended periods of time may cause bone marrow depression manifested as thrombocytopenia, leukopenia, and/or megaloblastic anemia. If signs of bone marrow depression occur, trimethoprim should be discontinued and the patient should be given leucovorin; 5 to 15 mg leucovorin daily has been recommended by some investigators.
Pharmacology
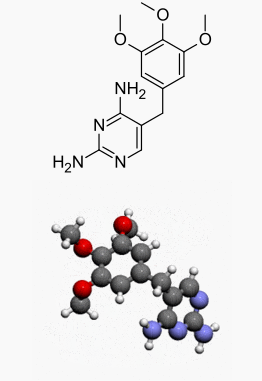
| |
Trimethoprim
| |
| Systematic (IUPAC) name | |
| 5-(3,4,5-Trimethoxybenzyl)pyrimidine-2,4-diamine | |
| Identifiers | |
| CAS number | |
| ATC code | J01 Template:ATCvet |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 290.32 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 90–100% |
| Protein binding | 44% |
| Metabolism | hepatic |
| Half life | 8-12 hours |
| Excretion | Urine (50–60%), faeces (4%) |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. | |
| Legal status |
Prescription Only (S4)(AU) ?(CA) POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral |
Mechanism of Action
Bacteriostatic lipophilic weak base structurally related to pyrimethamine, binds to and reversibly inhibits the bacterial enzyme dihydrofolate reductase, selectively blocking conversion of dihydrofolic acid to its functional form, tetrahydrofolic acid. This depletes folate, an essential cofactor in the biosynthesis of nucleic acids, resulting in interference with bacterial nucleic acid and protein production.
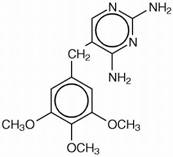
Structure
Trimethoprim is 5-[(3,4,5-trimethoxyphenyl)methyl]-2,4-pyrimidinediamine. It is a white to light yellow, odorless, bitter compound with a molecular weight of 290.32 and the molecular formula C14H18N4O3. The structural formula is:
Pharmacodynamics
There is limited information regarding Trimethoprim Pharmacodynamics in the drug label.
Pharmacokinetics
Trimethoprim is rapidly absorbed following oral administration. It exists in the blood as unbound, protein-bound, and metabolized forms. Ten to twenty percent of trimethoprim is metabolized, primarily in the liver; the remainder is excreted unchanged in the urine. The principal metabolites of trimethoprim are the 1- and 3-oxides and the 3'- and 4'-hydroxy derivatives. The free form is considered to be the therapeutically active form. Approximately 44% of trimethoprim is bound to plasma proteins.
Mean peak serum concentrations of approximately 1.0 mcg/mL occur 1 to 4 hours after oral administration of a single 100 mg dose. A single 200 mg dose will result in serum levels approximately twice as high. The half-life of trimethoprim ranges from 8 to 10 hours. However, patients with severely impaired renal function exhibit an increase in the half-life of trimethoprim, which requires either dosage regimen adjustment or not using the drug in such patients. During a 13 week study of trimethoprim administered at a daily dosage of 200 mg (50 mg q.i.d.), the mean minimum steady-state concentration of the drug was 1.1 mcg/mL. Steady-state concentrations were achieved within 2 to 3 days of chronic administration and were maintained throughout the experimental period.
Excretion of trimethoprim is primarily by the kidneys through glomerular filtration and tubular secretion. Urine concentrations of trimethoprim are considerably higher than are the concentrations in the blood. After a single oral dose of 100 mg, urine concentrations of trimethoprim ranged from 30 to 160 mcg/mL during the 0 to 4 hour period and declined to approximately 18 to 91 mcg/mL during the 8 to 24 hour period. A 200 mg single oral dose will result in trimethoprim urine levels approximately twice as high. After oral administration, 50% to 60% of trimethoprim is excreted in the urine within 24 hours, approximately 80% of this being unmetabolized trimethoprim.
Since normal vaginal and fecal flora are the source of most pathogens causing urinary tract infections, it is relevant to consider the distribution of trimethoprim into these sites. Concentrations of trimethoprim in vaginal secretions are consistently greater than those found simultaneously in the serum, being typically 1.6 times the concentrations of simultaneously obtained serum samples. Sufficient trimethoprim is excreted in the feces to markedly reduce or eliminate trimethoprim-susceptible organisms from the fecal flora.
Trimethoprim also passes the placental barrier and is excreted in human milk.
Nonclinical Toxicology
Carcinogenesis
Long-term studies in animals to evaluate carcinogenic potential have not been conducted with trimethoprim.
Mutagenesis
Trimethoprim was demonstrated to be nonmutagenic in the Ames assay. In studies at two laboratories, no chromosomal damage was detected in cultured Chinese hamster ovary cells at concentrations approximately 500 times human plasma levels; at concentrations approximately 1000 times human plasma levels in these same cells, a low level of chromosomal damage was induced at one of the laboratories. No chromosomal abnormalities were observed in cultured human leukocytes at concentrations of trimethoprim up to 20 times human steady-state plasma levels. No chromosomal effects were detected in peripheral lymphocytes of human subjects receiving 320 mg of trimethoprim in combination with up to 1600 mg of sulfamethoxazole per day for as long as 112 weeks.
Clinical Studies
There is limited information regarding Trimethoprim Clinical Studies in the drug label.
How Supplied
- Trimethoprim tablets 100 mg in bottles of 100.
- Trimethoprim tablets 200 mg in bottles of 100.
Storage
Store at 20° to 25°C (68° to 77°F)
Images
Drug Images

{{#ask: Page Name::Trimethoprim |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
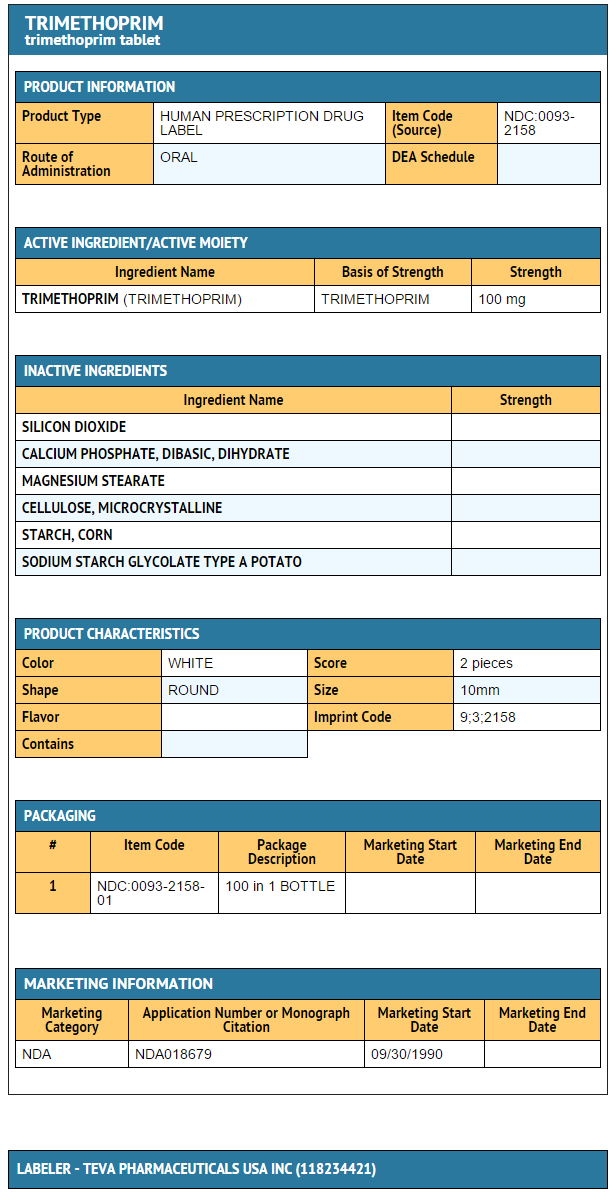
{{#ask: Label Page::Trimethoprim |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Patients should be counseled that antibacterial drugs including trimethoprim tablets, USP should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When trimethoprim tablets, USP are prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by trimethoprim tablets, USP or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with and without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Precautions with Alcohol
Alcohol-Trimethoprim interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Proloprim [1]
- Trimpex
Look-Alike Drug Names
There is limited information regarding Trimethoprim Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Trimethoprim |Label Name=Trimethoprim 100mg.png
}}