Thrombocytopenia: Difference between revisions
No edit summary |
|||
| Line 18: | Line 18: | ||
{{CMG}} | {{CMG}} | ||
==Overview== | |||
'''Thrombocytopenia''' (or '''-paenia''', or '''thrombopenia''' in short) is the presence of relatively few [[platelets]] in [[blood]]. | '''Thrombocytopenia''' (or '''-paenia''', or '''thrombopenia''' in short) is the presence of relatively few [[platelets]] in [[blood]]. | ||
| Line 442: | Line 441: | ||
{{reflist|2}} | {{reflist|2}} | ||
{{Hematology}} | {{Hematology}} | ||
| Line 451: | Line 446: | ||
[[Category:Blood disorders]] | [[Category:Blood disorders]] | ||
[[Category:Hematology]] | [[Category:Hematology]] | ||
Revision as of 20:25, 8 August 2011
| Thrombocytopenia | |
 | |
|---|---|
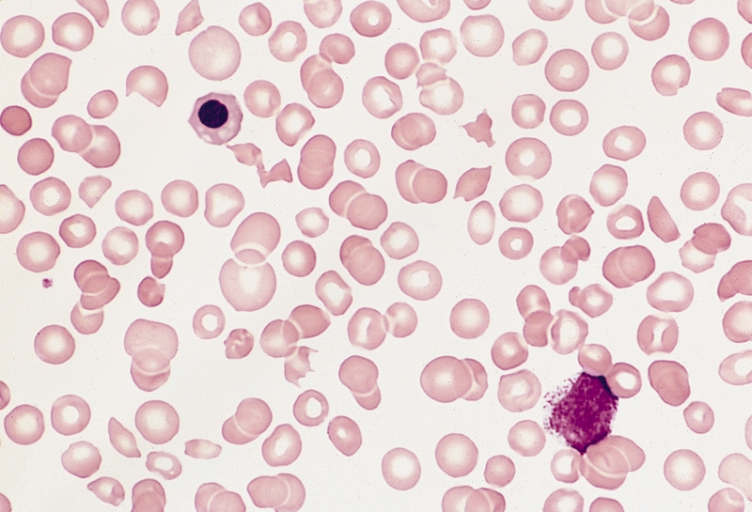
| Marked thrombocytopenia and fragmented red blood cells. Image courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology | |
| ICD-10 | D69.6, P61.0 |
| ICD-9 | 287.3, 287.4, 287.5 |
| OMIM | 188000 313900 |
| DiseasesDB | 27522 |
| MedlinePlus | 000586 |
| MeSH | D013921 |
|
WikiDoc Resources for Thrombocytopenia |
|
Articles |
|---|
|
Most recent articles on Thrombocytopenia Most cited articles on Thrombocytopenia |
|
Media |
|
Powerpoint slides on Thrombocytopenia |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Thrombocytopenia at Clinical Trials.gov Trial results on Thrombocytopenia Clinical Trials on Thrombocytopenia at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Thrombocytopenia NICE Guidance on Thrombocytopenia
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Thrombocytopenia Discussion groups on Thrombocytopenia Patient Handouts on Thrombocytopenia Directions to Hospitals Treating Thrombocytopenia Risk calculators and risk factors for Thrombocytopenia
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Thrombocytopenia |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Thrombocytopenia (or -paenia, or thrombopenia in short) is the presence of relatively few platelets in blood.
Generally speaking a normal platelet count ranges from 150,000 and 450,000 per mm3. These limits, however, are determined by the 2.5th lower and upper percentile, and a deviation does not necessarily imply any form of disease. The number of platelets in a blood sample also decreases rather quickly with time and a low platelet count may be caused by a delay between sampling and analysis.
Signs and symptoms
Often, low platelet levels do not lead to clinical problems; rather, they are picked up on a routine full blood count (or CBC, complete blood count ). Occasionally, there may be bruising, particularly purpura in the forearms, nosebleeds and/or bleeding gums.
It is vital that a full medical history is elicited, to ensure the low platelet count is not due to a secondary process. It is also important to ensure that the other blood cell types red blood cells, and white blood cells, are not also suppressed.
Diagnosis
Laboratory tests might include: full blood count, liver enzymes, renal function, vitamin B12 levels, folic acid levels, erythrocyte sedimentation rate, and peripheral blood smear.
If the cause for the low platelet count remains unclear, bone marrow biopsy is often undertaken, to differentiate whether the low platelet count is due to decreased production or peripheral destruction.
Causes
There are two broad mechanisms of thrombocytopenia: reduced platelet production and increased platelet destruction. Thormbocytopenia is seen in a variety of infectious and genetic disorders as well as a side effect of a large list of phramacotherapies.
Decreased production
Vitamin deficiencies
- Vitamin B12 deficiency
- Folic acid deficiency
- Iron deficiency
Hematologic disorders
- Pancytopenia
- Aplastic anemia
- Acute lymphoblastic leukemia
- Acute myeloid leukemia
- Chronic lymphocytic leukaemia
- Chronic myeloid leukaemia
- Hairy cell leukaemia
- Myeloma
- Non-Hodgkin lymphoma
- Myelodysplastic syndrome
- Myelofibrosis
Reduced thrombopoiesis due to reduced thrombopoietin production
- Decreased production of thrombopoietin by the liver in liver failure.
Infectious etiologies
- Sepsis, systemic viral or bacterial infection
- Dengue fever can cause thrombocytopenia by direct infection of bone marrow megakaryocytes as well as immunological shortened platelet survival
- Protozoa and protozoal conditions
- Visceral leishmaniasis
- Human granulocytic ehrlichiosis
- Human monocytotropic ehrlichiosis
- Mycoplasma pneumonia
- Staphylococcal toxic shock syndrome
- Epstein-Barr virus
- Hantavirus
- HIV-1 disease
- Infectious mononucleosis
- Lassa fever
- Measles
- Mumps
- Oklahoma tick fever
- Rubella
- Severe acute respiratory distress syndrome
- Tick born encephalitis
Intrauterine acquired conditions
Hereditary syndromes
- Congenital Amegakaryocytic Thrombocytopenia (CAMT)
- Bernard-Soulier syndrome, associated with large platelets
- May-Hegglin anomaly, the combination of thrombocytopenia, pale-blue leuckocyte inclusions, and giant platelets
- Grey platelet syndrome
- Alport syndrome
Chromosomal abnormalities
Mendelian inherited conditions
- Autoimmune lymphoproliferative syndrome type 1
- Autoimmune lymphoproliferative syndrome type 2
- Radial aplasia-thrombocytopenia syndrome or Thrombocytopenia absent radius syndrome
- von Willebrand disease, platelet type
Autosomal dominant conditions
- Arias oculootoradial syndrome
- Complement factor H deficiency
- Fechtner syndrome
- May-Hegglin anomaly
- Platelet glycoprotein 4 deficiency
- Sebastian platelet syndrome
Autosomal recessive conditions
- Chediak-Higashi disease
- Dibasic aminoaciduria type 2
- Familial histiocytic reticulosis
- Fanconi anaemia
- Folate malabsorption hereditary
- Gaucher disease
- Griscelli syndrome type 1
- Histiocytosis X
- Holocarboxylase synthase deficiency
- Iminodipeptiduria
- Isovaleric acidaemia
- Methylmalonic aciduria type 2
- Neuroectodermal melanolysosomal disease
- Niemann-Pick disease type B
- Omenn syndrome
- Platelet glycoprotein Ib deficiency
- Propionyl-CoA carboxylase deficiency PCCA type
- Sea blue histiocytosis
- Shwachman-Diamond syndrome
X-linked inherited conditions
- GATA1-related cytopenia
- Immunodysregulation polyendocrinopathy and enteropathy, X-linked
- Wiskott-Aldrich syndrome
- X-linked hyperimmunoglobulin M syndrome
- Mitochondrial genome inherited conditions
- MELAS
Chemical exposure
Strontium-89
Zinc
Increased destruction
Hematologic Disorders
- Idiopathic thrombocytopenic purpura (ITP)
- Thrombotic thrombocytopenic purpura (TTP)
- Hemolytic-uremic syndrome (HUS)
- Disseminated intravascular coagulation (DIC)
- Paroxysmal nocturnal hemoglobinuria (PNH)
- Neonatal alloimmune thrombocytopenia (NAITP)
- Following transfusion or post transfusion alloimmune thrombocytopenia
- Evans syndrome
- Macrophage activation syndrome
Cardiovascular causes
- Cholesterol embolism
- Intraaortic balloon pump placement
- Endocarditis
Obstetric disorders
Autoimmunde Disorders
Infectious Disorders
- Dengue fever has been shown to cause shortened platelet survival and immunological platelet destruction
- HIV [2]
Other disorders
- Splenic sequestration of platelets due to hypersplenism
Medication-induced
Thrombocytopenia associated with medications can be due to either a reduction in production of platelets or increased destruction.
Mechanisms
Direct myelosuppression
- Valproic acid
- Methotrexate
- Carboplatin
- Interferon
- Other chemotherapy drugs
Immunological platelet destruction
- Drug binds Fab portion of an antibody. The classic example of this mechanism is the quinidine group of drugs. The Fc portion of the antibody molecule is not involved in the binding process.
- Drug binds to Fc, and drug-antibody complex binds and activates platelets. Heparin induced thrombocytopenia (HIT) is the classic example of this phenomenon. In HIT, the heparin-antibody-platelet factor 4 (PF4) complex binds to Fc receptors on the surface of the platelet. Since Fc portion of the antibody is bound to the platelets, they are not available to the Fc receptors of the reticulo-endothelial cells, so therefore this system cannot destroy platelets as usual. This may explain why severe thrombocytopenia is not a common feature of HIT.
Heparin-induced thrombocytopenia
(HIT or white clot syndrome): this is a rare but serious condition that may occur in a hospitalized population. The most common clinical setting for HIT is in postoperative coronary artery bypass graft recipients, who may receive large quantities of heparin during surgery. HIT typically occurs about a week after exposure to heparin. The heparin-PF4 antibody complex will activate the platelets, and this can often lead to thrombosis. The term HITT, where the last T stands for thrombosis, denotes the concept that heparin-induced thrombocytopenia often is associated with thrombosis.
List of potential etiologies:
|
Sulphonamides Drugs, hormones and mediators Aclarubicin Anazolene Antithymocyte globulin Arsenic trioxide
|
Guanidinium Haem arginate Methyldopate
|
Para-amino salicylic acid Sulphasalazine Sunitinib malate |
Epiphenomenon
Kasabach-Merritt syndrome Paraneoplastic syndrome
Treatment
Treatment is guided by etiology and disease severity. The main concept in treating thrombocytopenia is to eliminate the underlying problem, whether that means discontinuing suspected drugs that cause thrombocytopenia, or treating underlying sepsis. Diagnosis and treatment of serious thrombocytopenia is usually directed by a hematologist.
Specific treatment plans often depend on the underlying etiology of the thrombocytopenia.
Thrombotic thrombocytopenic purpura (TTP)
Treatment of thrombotic thrombocytopenic purpura is a medical emergency, since the hemolytic anemia and platelet activation can lead to renal failure and changes in the level of consciousness. Treatment of TTP was revolutionized in the 1980s with the application of plasmapheresis. According to the Furlan-Tsai hypothesis [1] [2] , this treatment theoretically works by removing antibodies directed against the von Willebrand factor cleaving protease, ADAMTS-13. The plasmapheresis procedure also adds active ADAMTS-13 protease proteins to the patient, restoring a more physiological state of von Willebrand factor multimers. Patients with persistent antibodies against ADAMTS-13 do not always manifest TTP, and these antibodies alone are not sufficient to explain the how plasmapheresis treats TTP.
ITP
In many cases, ITP is self-limited, and does not require treatment. Platelet counts less than ten thousand per mm3 usually require treatment(less than fifty thousand requires treatment, less than ten thousand is a potentially dangerous situation) and patients with significant bleeding and thrombocytopenia due to ITP are also usually treated. The threshold for treating ITP has decreased since the 1990s, and hematologists recognize that patients rarely bleed with platelet counts greater than ten thousand, though there are documented exceptions to this observation. Treatments for ITP include:
Thrombopoetin analogues have been tested extensively for the treatment of ITP. These agents had previously shown promise but had been found to stimulate antibodies against endogenous thrombopoeitin or lead to thrombosis.
A medication known as AMG 531 was found to be safe and effective for the treatment of ITP in refractory patients. [3] AMG 531 is a peptide that bears no sequence homology with endogenous human thrombopoeitin, so it is not as likely to lead to neutralizing antibodies as previous peptide thrombopoeitin analogues. [4]
Heparin-induced thrombocytopenia and thrombosis (HITT)
Discontinuation of heparin is critical in a case of HITT. Beyond that, however, care must be taken to avoid a thrombosis, and patients started directly on warfarin after a diagnosis of HITT are at excess risk of venous limb gangrene. For this reason, patients are usually treated with a type of blood thinner called a direct thrombin inhibitor such as the FDA-approved lepirudin or argatroban. Other blood thinners sometimes used in this setting that are not FDA-approved for treatment of HITT include bivalirudin and fondaparinux. Platelet transfusions are not a routine component of the treatment of HITT, since thrombosis, not bleeding, is the usual associated problem in this illness.
Congenital amegakaryocytic thrombocytopenia (CAMT)
Bone Marrow/Stem Cell Transplant is the only thing that ultimately cures this genetic disease. Frequent platelet transfusions are required to keep the patient from bleeding to death until transplant is done.
Prognosis and Clinical Significance
In acute coronary syndrome trials and ST elevation MI trials, thrombocytipenia has been associated with an increase risk of major bleeding, transfusion, recurrent MI, stroke and both in-hosptial and 30 day mortality [5][6][7][8]
References
- ↑ Furlan M, Lämmle B (2001). "Aetiology and pathogenesis of thrombotic thrombocytopenic purpura and haemolytic uraemic syndrome: the role of von Willebrand factor-cleaving protease". Best Pract Res Clin Haematol. 14 (2): 437–54. PMID 11686108.
- ↑ Tsai H (2003). "Advances in the pathogenesis, diagnosis, and treatment of thrombotic thrombocytopenic purpura". J Am Soc Nephrol. 14 (4): 1072–81. PMID 12660343.
- ↑ Bussel J, Kuter D, George J, McMillan R, Aledort L, Conklin G, Lichtin A, Lyons R, Nieva J, Wasser J, Wiznitzer I, Kelly R, Chen C, Nichol J (2006). "AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP". N Engl J Med. 355 (16): 1672–81. PMID 17050891.
- ↑ Broudy V, Lin N (2004). "AMG531 stimulates megakaryopoiesis in vitro by binding to Mpl". Cytokine. 25 (2): 52–60. PMID 14693160.
- ↑ Gore JM, Spencer FA, Gurfinkel EP, López-Sendón J, Steg PG, Granger CB, FitzGerald G, Agnelli G (2009). "Thrombocytopenia in patients with an acute coronary syndrome (from the Global Registry of Acute Coronary Events [GRACE])". The American Journal of Cardiology. 103 (2): 175–80. doi:10.1016/j.amjcard.2008.08.055. PMID 19121432. Retrieved 2010-06-30. Unknown parameter
|month=ignored (help) - ↑ Merlini PA, Rossi M, Menozzi A, Buratti S, Brennan DM, Moliterno DJ, Topol EJ, Ardissino D (2004). "Thrombocytopenia caused by abciximab or tirofiban and its association with clinical outcome in patients undergoing coronary stenting". Circulation. 109 (18): 2203–6. doi:10.1161/01.CIR.0000127867.41621.85. PMID 15117843. Retrieved 2010-06-30. Unknown parameter
|month=ignored (help) - ↑ Nikolsky E, Sadeghi HM, Effron MB, Mehran R, Lansky AJ, Na Y, Cox DA, Garcia E, Tcheng JE, Griffin JJ, Stuckey TD, Turco M, Carroll JD, Grines CL, Stone GW (2005). "Impact of in-hospital acquired thrombocytopenia in patients undergoing primary angioplasty for acute myocardial infarction". The American Journal of Cardiology. 96 (4): 474–81. doi:10.1016/j.amjcard.2005.04.005. PMID 16098296. Retrieved 2010-06-30. Unknown parameter
|month=ignored (help) - ↑ Wang TY, Ou FS, Roe MT, Harrington RA, Ohman EM, Gibler WB, Peterson ED (2009). "Incidence and prognostic significance of thrombocytopenia developed during acute coronary syndrome in contemporary clinical practice". Circulation. 119 (18): 2454–62. doi:10.1161/CIRCULATIONAHA.108.827162. PMID 19398666. Retrieved 2010-06-30. Unknown parameter
|month=ignored (help)
Template:Hematology
Template:SIB
de:Thrombozytopenie hr:Trombocitopenija id:Trombositopenia it:Piastrinopenia mk:Тромбоцитопенија nl:Trombocytopenie sv:Trombocytopeni