Renal cell carcinoma
For patient information click here
Template:DiseaseDisorder infobox
|
Kidney cancer Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Renal cell carcinoma On the Web |
|
American Roentgen Ray Society Images of Renal cell carcinoma |
Steven C. Campbell, M.D., Ph.D.
Contributors: C. Michael Gibson, M.S., M.D., Cafer Zorkun M.D., PhD.
Please Join in Editing This Page and Apply to be an Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Synonyms and Key Words: RCC, renal cell, renal cell CA, renal cell carcinoma, kidney cancer, kidney carcinoma, kidney CA, Grawitz tumor, hypernephroma
Overview
Historical Perspective
Pathophysiology
Epidemiology & Demographics
Risk Factors
Screening
Causes
Differentiating Kidney cancer
Complications, & Prognosis
Risk factors
A history of smoking greatly increases the risk for developing renal cell carcinoma.
Genetic risk factors
Some people may also have inherited an increased risk to develop renal cell carcinoma, and a family history of kidney cancer increases the risk. People with von Hippel-Lindau disease, a hereditary disease that also affects the capillaries of the brain, commonly also develop renal cell carcinoma.
Autosomal dominant conditions
- Birt-Hogg-Dube syndrome
- Hereditary leiomyomatosis and renal cell cancer
- Hereditary nonpolyposis colorectal cancer
- von Hippel-Lindau syndrome
Autosomal recessive conditions
Classification
Recent genetic studies have altered the approaches used in classifying renal cell carcinoma. The following system can be used to classify these tumors:[1][2][3]
- Clear cell carcinoma (VHL and others on chromosome 3)
- Papillary carcinoma (MET, PRCC)
- Chromophobe renal carcinoma
- Collecting duct carcinoma
Other associated genes include TRC8, OGG1, HNF1A, HNF1B, TFE3, RCCP3, and RCC17.
Robson Staging of Renal cell carcinoma
- Stage I: Tumor confined within capsule of kidney
- Stage II: Tumor invading perinephric fat but confined within the Gerota fascia
- Stage III:
- A: Tumor invading the renal vein/IVC
- B: Tumor in regional lymph nodes
- C: Tumor with both renal vein and region lymph node involvement.
- Stage IV: Tumor invading adjacent viscera, excluding ipsilateral adrenal, or distant metastases
Diagnosis
Signs and symptoms
The classic triad is hematuria (blood in the urine), flank pain and an abdominal mass. This "classic triad" is infrequently present when the patient first presents for medical attention.
Other signs may include:
- Abnormal urine color (dark, rusty, or brown) due to blood in the urine (found in 60% of cases)
- Loin or groin pain (found in 40% of cases)
- Abdominal mass (25% of cases)
- Malaise, weight loss or anorexia (30% of cases)
- Polycythemia (5% of cases)
- Anemia resulting from depression of erythropoietin (5% of cases)
- The presenting symptom may be due to metastatic disease, such as a pathologic fracture of the hip due to a metastasis to the bone
- Enlargement of one testicle known as varicocele (usually the left, due to blockage of the left gonadal vein by tumor invasion of the left renal vein -- the right gonadal vein drains directly into the inferior vena cava)
- Hirsutism - Excessive hair growth (females)
- Constipation
- Hypertension (high blood pressure) resulting from secretion of renin by the tumour (30% of cases)
- Elevated calcium levels (Hypercalcemia)
- Paraneoplastic disease
- Renal arteriovenous malformation
- Renal cysts
- Pyrexia of unknown origin
- Renal enlargement
- AA amyloidosis
- Cerebral metastases
- Cutaneous metastasis
- Lung metastases
Diagnostic Findings
Pathology
Gross examination shows a hypervascular lesion in the renal cortex, which is frequently multilobulated, yellow (because of the lipid accumulation) and calcified.
Light microscopy shows tumor cells forming cords, papillae, tubules or nests, and are atypical, polygonal and large. Because these cells accumulate glycogen and lipids, their cytoplasm appear "clear", lipid-laden, the nuclei remain in the middle of the cells, and the cellular membrane is evident. Some cells may be smaller, with eosinophilic cytoplasm, resembling normal tubular cells. The stroma is reduced, but well vascularized. The tumor grows in large front, compressing the surrounding parenchyma, producing a pseudocapsule.[4]
Secretion of vasoactive substances (e.g. renin) may cause arterial hypertension, and release of erythropoietin may cause polycythemia (increased production of red blood cells).
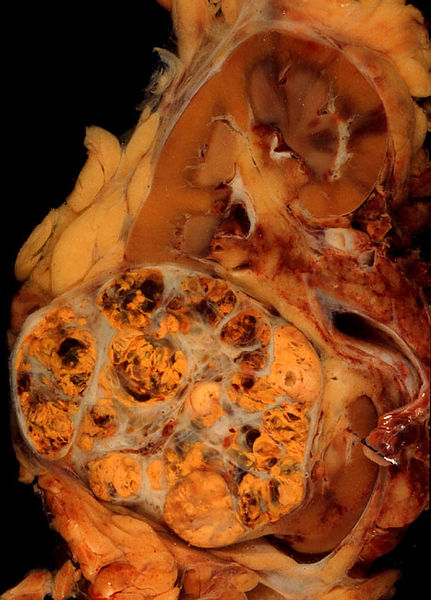
Radiology
The characteristic appearance of renal cell carcinoma (RCC) is a solid renal lesion which disturbs the renal contour. It will frequently have an irregular or lobulated margin. 85% of solid renal masses will be RCC. 10% of RCC will contain calcifications, and some contain macroscopic fat (likely due to invasion and encasement of the perirenal fat). Following intravenous contrast administration (computed tomography or magnetic resonance imaging), enhancement will be noted, and will increase the conspicuity of the tumor relative to normal renal parenchyma.
Patient #1
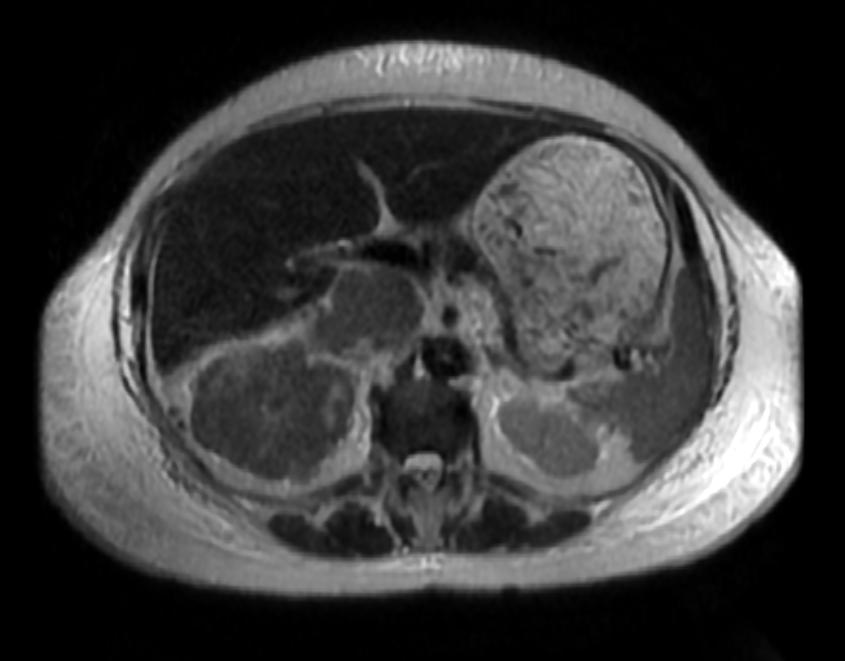
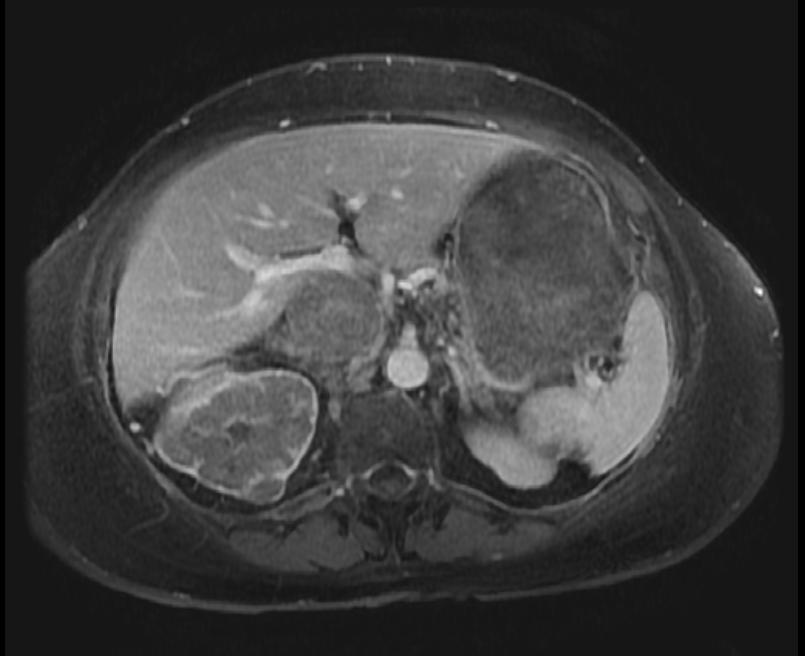
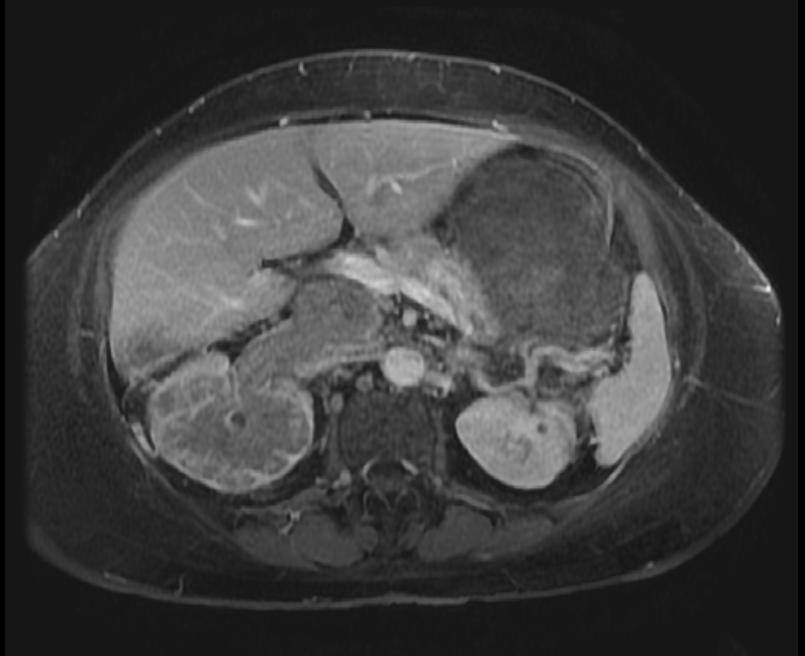
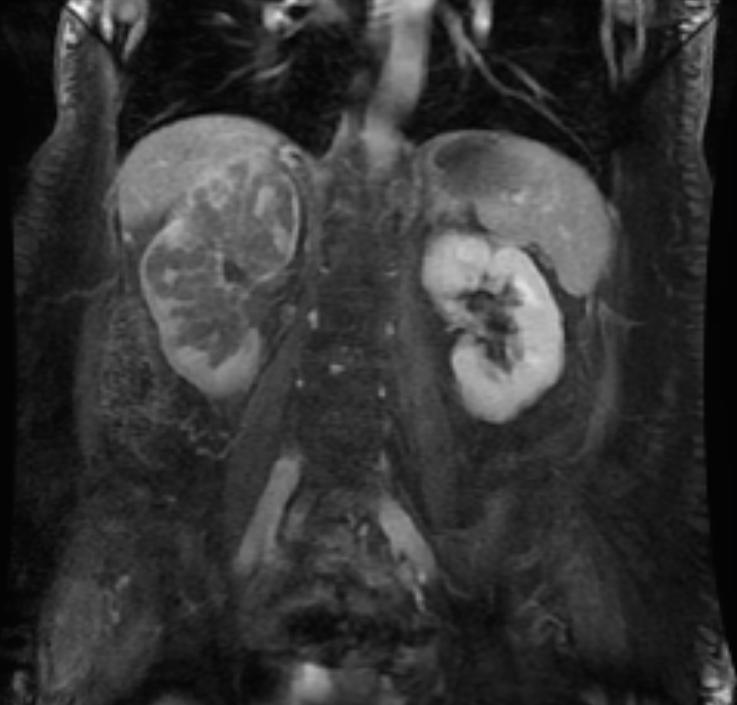
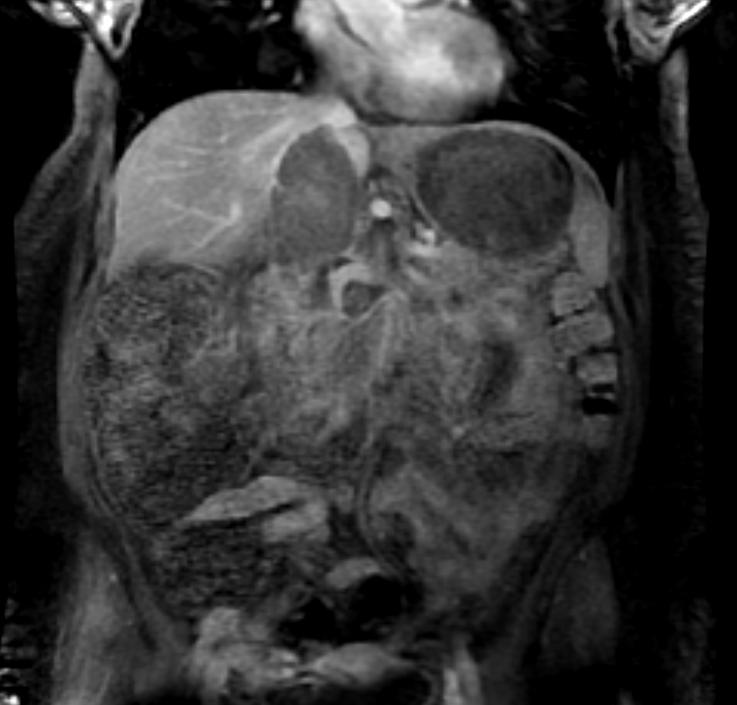
Patient #2
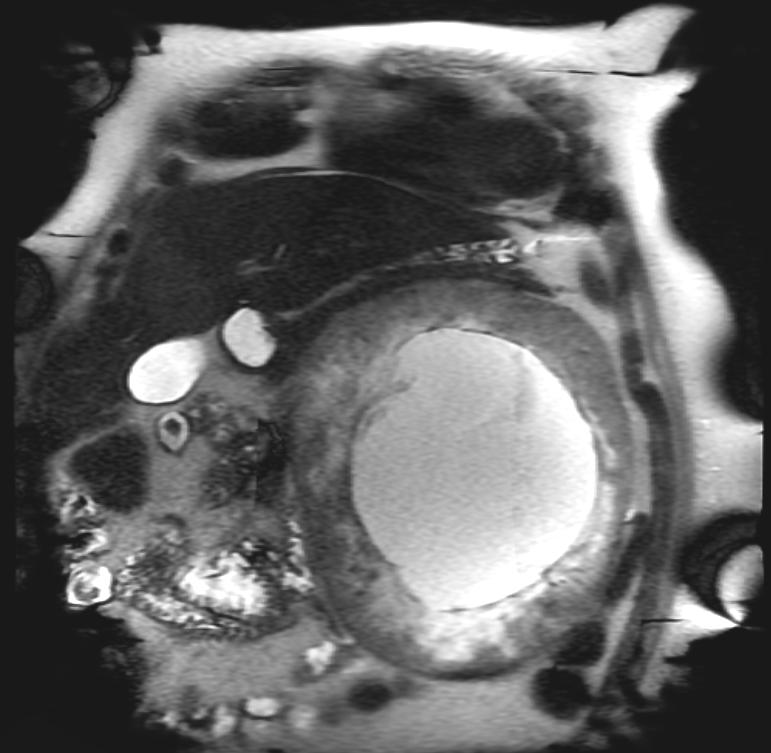
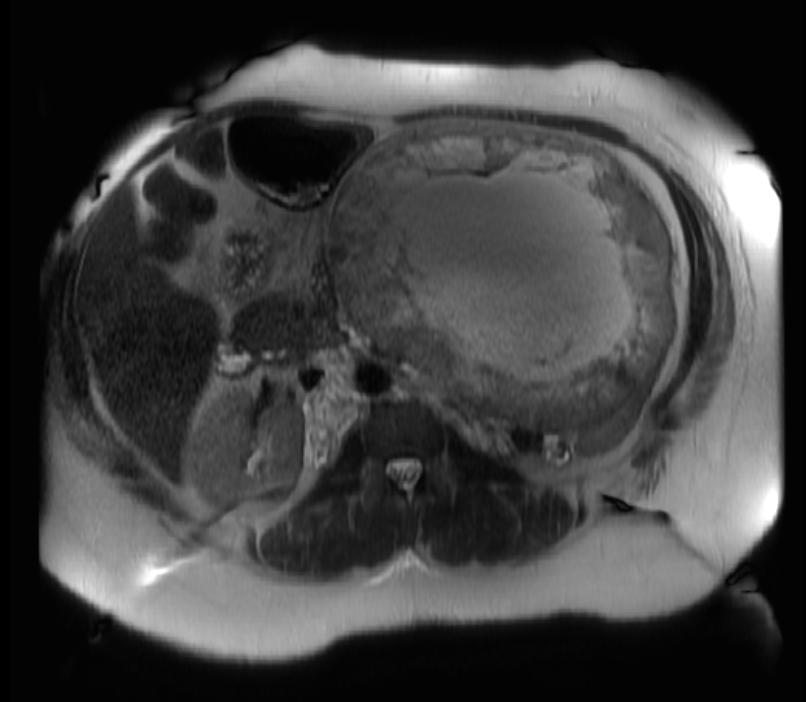
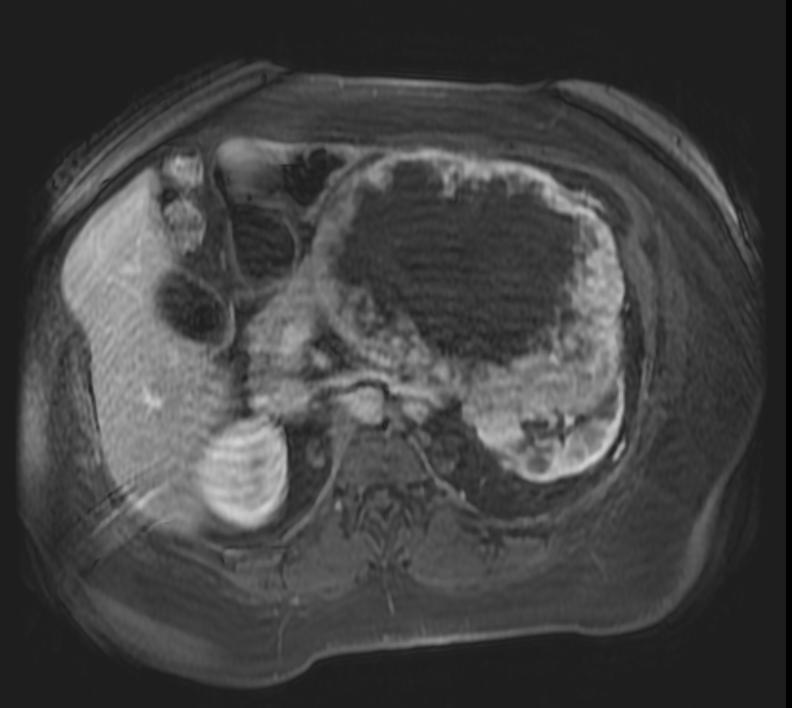
Patient #3
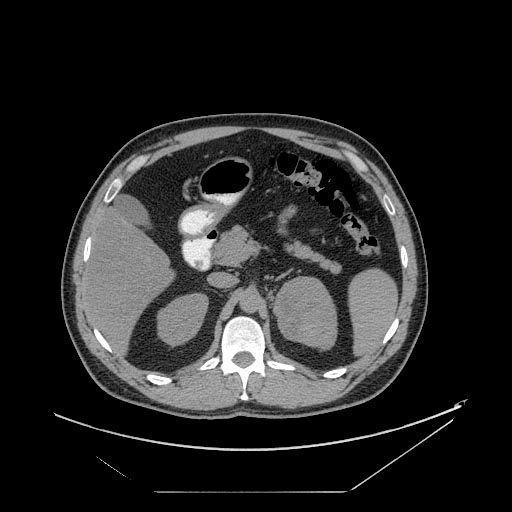
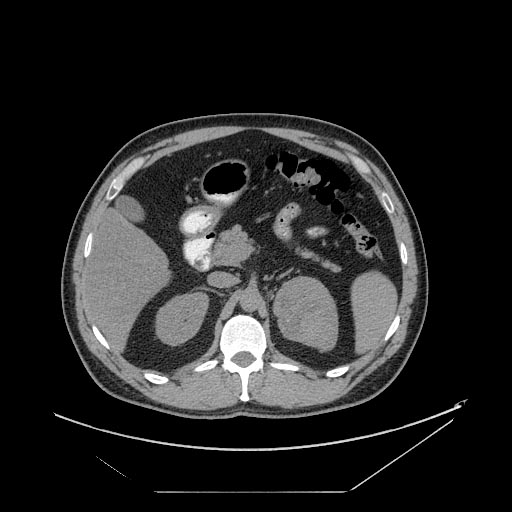
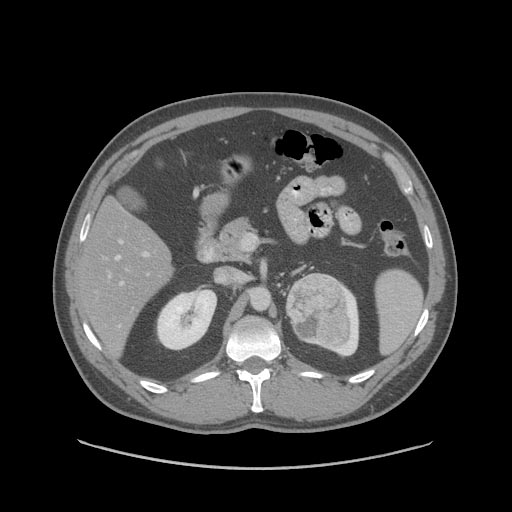
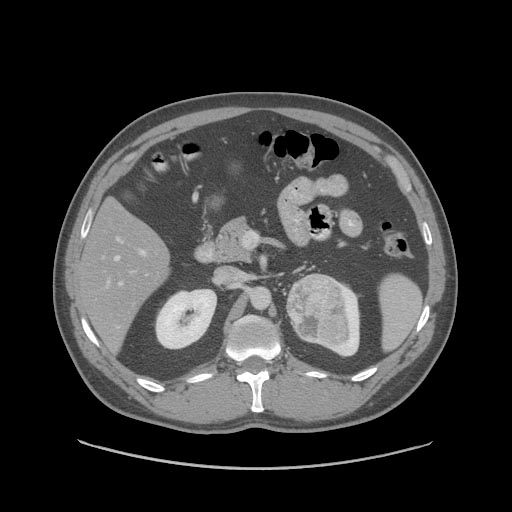
Patient #4
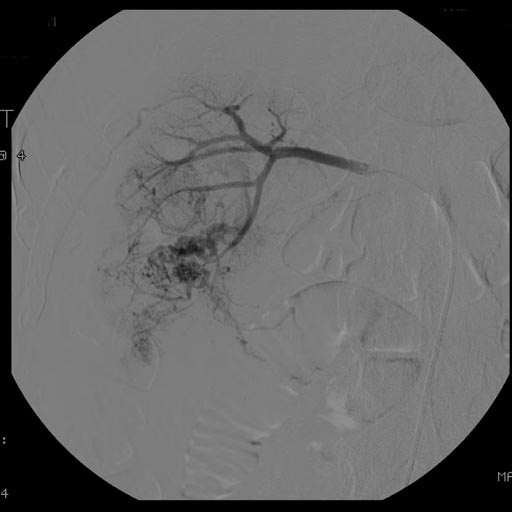
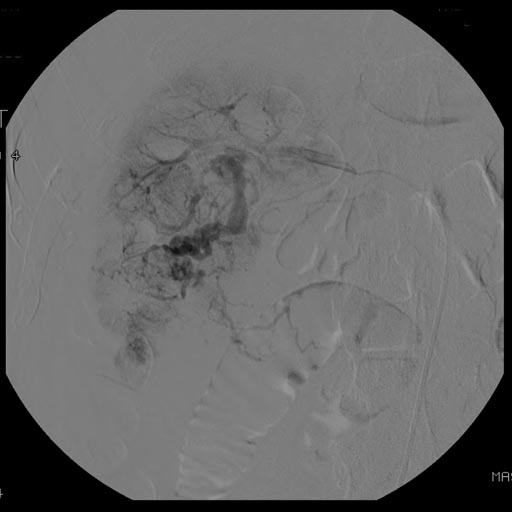
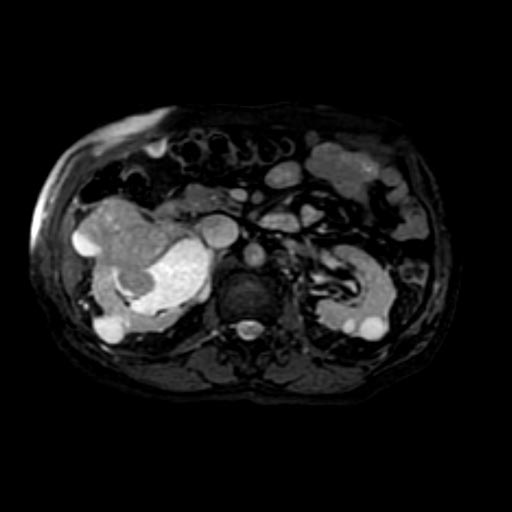
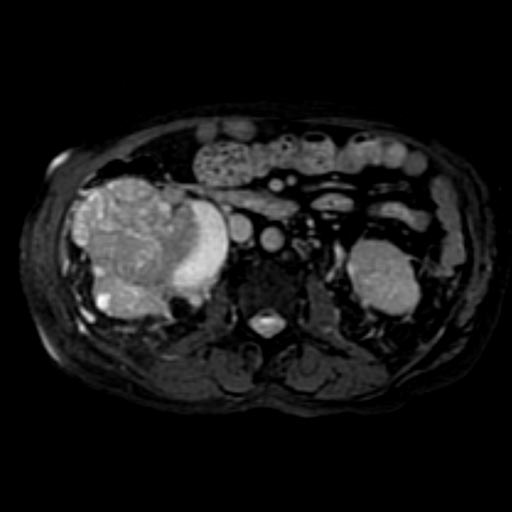

At diagnosis, 30% of renal cell carcinoma has spread to that kidney's renal vein, and 5-10% has continued on into the inferior vena cava[5].
Percutaneous biopsy can be performed by a radiologist using ultrasound or computed tomography to guide sampling of the tumor for the purpose of diagnosis. However this is not routinely performed because when the typical imaging features of renal cell carcinoma are present, the possibility of an incorrectly negative result together with the risk of a medical complication to the patient make it unfavorable from a risk-benefit perspective.This is not completely accurate, there are new experimental treatments.
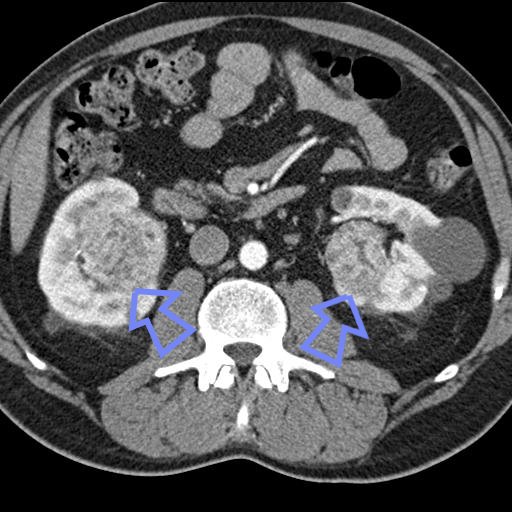
Complications
Patients with renal cell carcinoma are at risk of hepatic vein thrombosis aka/or Budd-Chiari syndrome
Treatment
If the tumor is confined to the kidneys (occurs in about 40% of cases), then RCC can be treated roughly 90% of the time with surgery. If RCC has spread outside of the kidneys, often into the lymph nodes or the main vein of the kidney, then it is often treated with chemotherapy and other treatments.
Watchful waiting
Small renal tumors represent the majority of tumors that are treated today by way of partial nephrectomy. The average growth of these masses is about 4-5 mm per year, and a significant proportion (up to 40%) of tumors less than 4cm in diameter are benign. More centers of excellence are incorporating needle biopsy to confirm the presence of malignant histology prior to recommending definitive surgical extirpation. In the elderly, patients with co-morbidities and in poor surgical candidates, small renal tumors may be monitored carefully with serial imaging. Most clinicians conservatively follow tumors up to a size threshold between 3-5 cm, beyond which the risk of distant spread (metastases) is about 5%.
Surgery
Surgical removal of all or part of the kidney (nephrectomy) is recommended. This may include removal of the adrenal gland, retroperitoneal lymph nodes, and possibly tissues involved by direct extension (invasion) of the tumor into the surrounding tissues. In cases where the tumor has spread into the renal vein, inferior vena cava, and possibly the right atrium (angioinvasion), this portion of the tumor can be surgically removed, as well. In case of metastases surgical resection of the kidney ("cytoreductive nephrectomy") may improve survival[6], as well as resection of a solitary metastatic lesion.
Percutaneous therapies
Percutaneous, image-guided therapies, usually managed by radiologists, are being offered to patients with localized tumor, but who are not good candidates for a surgical procedure. This sort of procedure involves placing a probe through the skin and into the tumor using real-time imaging of both the probe tip and the tumor by computed tomography, ultrasound, or even magnetic resonance imaging guidance, and then destroying the tumor with heat (radiofrequency ablation) or cold (cryotherapy). These modalities are at a disadvantage compared to traditional surgery in that pathologic confirmation of complete tumor destruction is not possible.
Radiation therapy
Radiation therapy is not commonly used for treatment of renal cell carcinoma because it is usually not successful. Radiation therapy may be used to palliate the symptoms of skeletal metastases.
Medications
RCC "elicits an immune response, which occasionally results in dramatic spontaneous remissions." This has encouraged a strategy of using immunomodulating therapies, such as cancer vaccines and interleukin-2 (IL-2), to reproduce this response. IL-2 has produced "durable remissions" in a small number of patients, but with substantial toxicity. Another strategy is to restore the function of the VHL gene, which is to destroy proteins that promote inappropriate vascularization. Bevacizumab, an antibody to VEGF, has significantly prolonged time to progression, but phase 3 trials have not been published. Sunitinib (Sutent), sorafenib (Nexavar), and temsirolimus, which are small-molecule inhibitors of proteins, have been approved by the U.S. F.D.A.[7]
Sorafenib was FDA approved in December 2005 for treatment of advanced renal cell cancer, the first receptor tyrosine kinase (RTK) inhibitor indicated for this use.
A month later, Sunitinib was approved as well. Sunitinib—an oral, small-molecule, multi-targeted (RTK) inhibitor—and sorafenib both interfere with tumor growth by inhibiting angiogenesis as well as tumor cell proliferation. Sunitinib appears to offer greater potency against advanced RCC, perhaps because it inhibits more receptors than sorafenib. However, these agents have not been directly compared against one another in a single trial. [2][3]
Recently the first Phase III study comparing an RTKI with cytokine therapy was published in the New England Journal of Medicine. This study showed that Sunitinib offered superior efficacy compared with interferon-α. Progression-free survival (primary endpoint) was more than doubled. The benefit for sunitinib was significant across all major patient subgroups, including those with a poor prognosis at baseline. 28% of sunitinib patients had significant tumor shrinkage compared with only 5% of patients who received interferon-α. Although overall survival data are not yet mature, there is a clear trend toward improved survival with sunitinib. Patients receiving sunitinib also reported a significantly better quality of life than those treated with IFNa. [8] Based on these results, lead investigator Dr. Robert Motzer announced at ASCO 2006 that “Sunitinib is the new reference standard for the first-line treatment of mRCC.” [9]
Temsirolimus (CCI-779) is an inhibitor of mTOR kinase (mamallian target of rapamycin) that was shown to prolong overall survival vs. interferon-α in patients with previously untreated metastatic renal cell carcinoma with three or more poor prognostic features. The results of this Phase III randomized study were presented at the 2006 annual meeting of the American Society of Clinical Oncology (www.ASCO.org).
Chemotherapy
Chemotherapy may be used in some cases, but cure is unlikely unless all the cancer can be removed with surgery. The use of Tyrosine Kinase (TK) inhibitors, such as Sunitinib and Sorafenib, and Temsirolimus are described in a different section.
Vaccine
In November 2006, it was announced that a vaccine had been developed and tested with very promising results.(See [4]) The new vaccine, called TroVax, works by harnessing the patient's own immune system to fight the disease. Oxford BioMedica[5]. Further vaccine trials are underway.
Cryoablation
This involves destroying the kidney tumor without surgery, by freezing the tumor. The process can remove 95% of tumors in one treatment and can be tolerated by patients who are not good candidates for surgery (older or weak patients). [10].
The outcome varies depending on the size of the tumor, whether it is confined to the kidney or not, and the presence or absence of metastatic spread. The Fuhrman grading, which measures the aggressiveness of the tumor, may also affect survival, though the data is not as strong to support this.
The five year survival rate is around 90-95% for tumors less than 4 cm. For larger tumors confined to the kidney without venous invasion, survival is still relatively good at 80-85%. For tumors that extend through the renal capsule and out of the local fascial investments, the survivability reduces to near 60%. If it has metastasized to the lymph nodes, the 5-year survival is around 5 % to 15 %. If it has spread metastatically to other organs, the 5-year survival rate is less than 5 %.
For those that have tumor recurrence after surgery, the prognosis is generally poor. Renal cell carcinoma does not generally respond to chemotherapy or radiation. Immunotherapy, which attempts to induce the body to attack the remaining cancer cells, has shown promise. Recent trials are testing newer agents, though the current complete remission rate with these approaches are still low, around 12-20% in most series.
Prognosis
The outcome varies depending on the size of the tumor, whether it is confined to the kidney or not, and the presence or absence of metastatic spread. The Furhman grading, which measures the aggressiveness of the tumor, may also affect survival, though the data is not as strong to support this.
The five year survival rate is around 90-95% for tumors less than 4 cm. For larger tumors confined to the kidney without venous invasion, survival is still relatively good at 80-85%. For tumors that extend through the renal capsule and out of the local fascial investments, the survivability reduces to near 60%. If it has metastasized to the lymph nodes, the 5-year survival is around 5 % to 15 %. If it has spread metastatically to other organs, the 5-year survival rate is less than 5 %.
For those that have tumor recurrence after surgery, the prognosis is generally poor. Renal cell carcinoma does not generally respond to chemotherapy or radiation. Immunotherapy, which attempts to induce the body to attack the remaining cancer cells, has shown promise. Recent trials are testing newer agents, though the current complete remission rate with these approaches are still low, around 12-20% in most series.
See also
External links
- Photo at: Atlas of Pathology
- www.411cancer.com - General Cancer Information
- Vaccine for kidney cancer
References
- ↑ Reuter VE, Presti JC (2000). "Contemporary approach to the classification of renal epithelial tumors". Semin. Oncol. 27 (2): 124–37. PMID 10768592. Unknown parameter
|month=ignored (help) - ↑ Bodmer D, van den Hurk W, van Groningen JJ; et al. (2002). "Understanding familial and non-familial renal cell cancer". Hum. Mol. Genet. 11 (20): 2489–98. PMID 12351585. Unknown parameter
|month=ignored (help) - ↑ Cotran, Ramzi S.; Kumar, Vinay; Fausto, Nelson; Nelso Fausto; Robbins, Stanley L.; Abbas, Abul K. (2005). Robbins and Cotran pathologic basis of disease. St. Louis, Mo: Elsevier Saunders. p. 1016. ISBN 0-7216-0187-1.
- ↑ http://www.pathologyatlas.ro/Renal%20Clear%20Cell%20Carcinoma.html
- ↑ Oto A, Herts BR, Remer EM, Novick AC. Inferior vena cava tumor thrombus in renal cell carcinoma: staging by MR imaging and impact on surgical treatment. AJR Am J Roentgenol. 1998 Dec;171(6):1619-24. PMID 9843299.
- ↑ Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. 2004 Mar;171(3):1071-6. PMID 14767273.
- ↑ Michaelson MD, Iliopoulos O, McDermott DF, McGovern FJ, Harisinghani MG, Oliva E (2008). "Case records of the Massachusetts General Hospital. Case 17-2008. A 63-year-old man with metastatic renal-cell carcinoma". N Engl J Med. 358 (22): 2389–96. doi:10.1056/NEJMcpc0802449. PMID 18509125. Unknown parameter
|month=ignored (help) - ↑ Motzer RJ; et al. (2007). "Sunitinib versus interferon alfa in metastatic renal-cell carcinoma". N Engl J Med. 356 (2): 115–124. doi:10.1056/NEJMoa065044. PMID 17215529.
- ↑ Motzer RJ; et al. "Phase 3 Randomized Trial of Sunitinib malate (SU11248) versus Interferon-alfa as First-line Systemic Therapy for Patients with Metastatic Renal Cell Carcinoma". ASCO 2006.
- ↑ <http://www.yourcancertoday.com/news/drnakada.html" title=" Dr. Nakada, on Your Cancer Today
Template:Tumors Template:Nephrology Template:Tumor morphology Template:SIB
bs:Rak bubrega de:Nierenkrebs hr:Rak bubrega it:Cancro del rene fi:Munuaissyöpä sv:Njurcancer