Penicillamine
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Variable |
| Metabolism | Hepatic |
| Elimination half-life | 1 hour |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
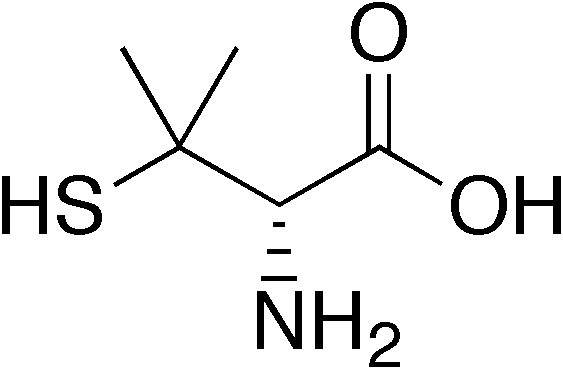
| Formula | C5H11NO2S |
| Molar mass | 149.212 g/mol |
Penicillamine is a pharmaceutical of the chelator class. It is sold under the trade names of Cuprimine and Depen. The pharmaceutical form is D-penicillamine, as L-penicillamine is toxic (it inhibits the action of pyridoxine). It is a metabolite of penicillin, although it has no antibiotic properties.
Uses
Penicillamine is used as a form of immunosuppression to treat rheumatoid arthritis. It works by reducing numbers of T-lymphocytes, inhibiting macrophage function, decreasing IL-1, decreasing rheumatoid factor, and preventing collagen from cross-linking.
Penicillamine is also used for treatment of scleroderma.
It is used as a chelating agent:
- In Wilson's disease, a rare genetic disorder of copper metabolism, penicillamine treatment relies on its binding to accumulated copper and elimination through urine.
- In cystinuria, a hereditary disorder featuring increased cystine excretion, penicillamine binds with the cystine to make it more soluble.
- Penicillamine has been used to treat mercury poisoning.
History
Dr John Walshe (1956) first described the use of penicillamine in Wilson's disease. He had discovered the compound in the urine of patients (including himself) who had taken penicillin, and experimentally confirmed that it increased urinary copper excretion by chelation. He had initial difficulty convincing several world experts of the time (Drs Denny Brown and Cumings) of its efficacy, as they held that Wilson's disease was not primarily a problem of copper homeostasis but of amino acid metabolism, and that dimercaprol should be used as a chelator. Later studies confirmed both the copper-centered theory and the efficacy of D-penicillamine. Walshe also pioneered other chelators in Wilson's such as triethylene tetramine 2HCl and tetrathiomolybdate (Walshe 2003).
References
- Walshe JM. Penicillamine, a new oral therapy for wilson's disease. Am J Med. 1956;21:487-95. PMID 13362281.
- Walshe JM. The story of penicillamine: a difficult birth. Mov Disord 2003;18:853-9. PMID 12889074.
External links
- Penicillamine (Systemic) - Medlineplus.org
- Penicillamine and Arthritis - Medicinenet.com
- Pages with script errors
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Amino acids
- Chelating agents
- Immunosuppressive agents
- Thiols