Paragonimus westermani: Difference between revisions
No edit summary |
No edit summary |
||
| (5 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
'''''Paragonimus westermani''''' is the | __NOTOC__ | ||
{{About0|Paragonimus infection}} | |||
{{Paragonimus infection}} | |||
{{CMG}} | |||
==Overview== | |||
'''''Paragonimus westermani''''' is the major species of lung fluke to infects humans, causing [[paragonimiasis]]. The species sometimes is called the '''Japanese Lung fluke''' or '''Oriental Lung fluke'''. Human infections are most common in eastern Asia and in South America. ''Paragonimus westermani'' was discovered when two Bengal tigers died of paragonimiasis in zoos in Europe in 1878. Several years later Infections in humans were recognised in [[Formosa]]. | |||
== Introduction == | |||
Paragonimiasis is a food-borne parasitic infection caused by the lung fluke. It may cause a [[Acute (medicine)|sub-acute]] to [[Chronic (medicine)|chronic]] inflammatory disease of the lung. It is one of the most familiar lung flukes with the widest geographical range. It was discovered by Coenraad Kerbert (1849-1927) in 1878. | |||
== Causative agent == | |||
More than 30 species of [[trematodes]] (flukes) of the genus ''Paragonimus'' have been reported to infect animals and humans. Among the more than 10 species reported to infect humans, the commonest is ''Paragonimus westermani'', the oriental lung fluke.<ref name=Markell2006 /><ref name=CDCpage2 /> | |||
== Morphology == | |||
[[File:Paragonimus_westermanii2.jpg|right|200px|thumb|''Paragonimus westermani''. An adult of the hermaphroditic generation.]] | |||
In size, shape, and color, ''Paragonimus westermani'' resembles a coffee bean when alive. Adult worms are 7.5 mm to 12 mm long and 4 mm to 6 mm wide. The thickness ranges from 3.5 mm to 5 mm. The skin of the worm ([[Integumentary system|tegument]]) is thickly covered with scalelike spines. The oral and ventral suckers are similar in size, with the latter placed slightly pre-equatorially. The excretory bladder extends from the posterior end to the [[pharynx]]. The lobed [[testes]] are adjacent from each other located at the posterior end, and the lobed [[ovaries]] are off-centered near the center of the worm (slightly postacetabular). The uterus is located in a tight coil to the right of the acetabulum, which is connected, to the vas deferens. The vitelline glands, which produce the yolk for the eggs, are widespread in the lateral field from the pharynx to the posterior end. Inspection of the tegumental spines and shape of the metacercariae may distinguish between the 30-odd species of ''Paragonimus spp.'' but the distinction is sufficiently difficult to justify suspicion that many of the described species are [[Synonym (taxonomy)|synonyms]].<ref name= "isbn0-697-26071-2">{{cite book |author=Janovy, John; Schmidt, Gerald D.; Roberts, Larry S. |title=Gerald D. Schmidt & Larry S. Roberts' Foundations of parasitology |publisher=Wm. C. Brown |location=Dubuque, Iowa |year=1996 |pages= |isbn=0-697-26071-2 |oclc= |doi= |accessdate=}}</ref> | |||
[[File:Paragonimus_westermani3.jpg|thumb|Egg of ''Paragonimus westermani'']] | |||
* Eggs: ''Paragonimus westermani'' eggs range from 80 to 120 µm long by 45 to 70 µm wide. They are yellow-brown, ovoid or elongate, with a thick shell, and often asymmetrical with one end slightly flattened. At the large end, the operculum is clearly visible. The opposite (abopercular) end is thickened. The eggs are [[Embryonated|unembryonated]] when passed in sputum or feces.<ref name=CDCpage2 /> | |||
* Cercaria (not shown): Cercariae are often indistinguishable between species. There is a large posterior sucker, and the exterior is spined. | |||
* Metacercaria: Metacercariae are usually encysted in tissue. The exterior is spined and has two suckers | |||
* Adults: Adult flukes are typically reddish brown and ovoid, measuring 7 to 16 mm by 4 to 8 mm, similar in size and appearance to a coffee bean.They are hermaphroditic, with a lobed ovary located anterior to two branching testes. Like all members of the Trematoda, they possess oral and ventral suckers. | |||
== History of discovery == | |||
''P. westermani'' was discovered in the lungs of a human by Ringer in 1879<ref name=Muller1996 /> and eggs in the sputum were recognized independently by Manson and Erwin von Baelz in 1880.<ref name=Muller1996 /><ref name=Manson1881 /> Manson proposed the snail as an intermediate host and various Japanese workers detailed the whole life cycle in the snail between 1916 and 1922.<ref name=Grove1990 /> The species name ''P. westermani'' was named after Pieter Westerman (1859-1925) a zookeeper who noted the trematode in a Bengal tiger in an Amsterdam Zoo.<ref name=Desowitz1987 /> | |||
== Life cycle == | |||
[[Embryonated|Unembryonated]] eggs are passed in the sputum of a human or feline. Two weeks later, [[miracidia]] develop in the egg and hatches. The miracidia penetrate its first [[intermediate host]] (snail). Within the snail mother sporocyst form and produce many mother rediae, which subsequently produce many daughter rediae which shed crawling cercariae into fresh water. The crawling cercariae penetrate fresh water crabs and encyst in its muscles becoming metacercaria. Humans or felines then eat the infected crabs raw. Once eaten, the metacercaria excysts and penetrates the gut, diaphragm and lung where it becomes an adult worm in pairs. | |||
The first intermediate hosts of the ''Paragonimus westermani'' are [[freshwater snail]]s: | |||
* ''Semisulcospira amurensis''<!--VERIFY CURRENT TAXONOMY OF Semisulcospira --><ref name="WHO 1995">[[World Health Organization]] (1995). ''Control of Foodborne Trematode Infection''. WHO Technical Report Series. 849. [http://whqlibdoc.who.int/trs/WHO_TRS_849_(part1).pdf PDF part 1], [http://whqlibdoc.who.int/trs/WHO_TRS_849_(part2).pdf PDF part 2]. page 125-126.</ref> | |||
* ''Semisulcospira calculus''<ref name="WHO 1995"/> | |||
* ''Semisulcospira cancellata''<ref name="WHO 1995"/> | |||
* ''Semisulcospira extensa''<ref name="WHO 1995"/> | |||
* ''Semisulcospira gottschei''<ref name="WHO 1995"/> | |||
* ''Semisulcospira libertina'' - synonym: ''Semisulcospira toucheana''<ref name="WHO 1995"/> | |||
* ''Semisulcospira mandarina'' - synonym: ''Semisulcospira wegckiangensis''<ref name="WHO 1995"/> | |||
* ''Semisulcospira multicincta''<ref name="WHO 1995"/> | |||
* ''Semisulcospira nodiperda''<ref name="WHO 1995"/> | |||
* ''Semisulcospira nodiperda quinaria''<ref name="WHO 1995"/> | |||
* ''Semisulcospira paucincta''<ref name="WHO 1995"/> | |||
* ''Semisulcospira peregrinomum''<ref name="WHO 1995"/> | |||
For many years ''[[Tarebia granifera]]'' was believed<ref name="WHO 1995"/> to be an intermediate host for the ''Paragonimus westermani'', but Michelson showed in 1992 that this was erroneous.<ref>{{cite journal | author = Michelson E | year = 1992 | title = ''Thiara granifera'': a victim of authoritarianism? | url = | journal = [[Malacological Review]] | volume = 25 | issue = | pages = 67–71 }}</ref><ref name="Appleton 2009"/> | |||
Paragonimus has a quite complex life-cycle that involves two intermediate hosts as well as humans. Eggs first develop in water after being expelled by coughing ([[Embryonated|unembryonated]]) or being passed in human feces. In the external environment, the eggs become embryonated. In the next stage, the parasite miracidia hatch and invades the first intermediate host such as a species of freshwater snail. Miracidia penetrate its soft tissues and go through several developmental stages inside the snail but mature into cercariae in 3 to 5 months. Cercariae next invade the second intermediate host such as crabs or crayfish and encyst to develop into metacercariae within 2 months. Infection of humans or other mammals (definitive hosts) occurs via consumption of raw or undercooked crustaceans. Human infection with P. westermani occurs by eating inadequately cooked or pickled crab or crayfish that harbor metacercariae of the parasite. The metacercariae excyst in the duodenum, penetrate through the intestinal wall into the peritoneal cavity, then through the abdominal wall and diaphragm into the lungs, where they become encapsulated and develop into adults. The worms can also reach other organs and tissues, such as the brain and striated muscles, respectively. However, when this takes place completion of the life cycles is not achieved, because the eggs laid cannot exit these sites.<ref name=CDCpage2 /> | |||
== Epidemiology == | |||
Reservoir hosts of ''Paragonimus'' spp. include numerous species of carnivores including felids, canids, viverrids, mustelids, some rodents and pigs. Humans become infected after eating raw [[freshwater crab]]s or [[crayfish]] that have been encysted with the metacerciaria. Southeast Asia is more predominately more infected because of lifestyles. Raw seafood is popular in these countries. Crab collectors string raw crabs together and bring them miles inland to sell in Taiwan markets. These raw crabs are then marinated or pickled in vinegar or wine to coagulate the crustacean muscle. This method of preparation does not kill the metacercariae, consequently infecting the host. Smashing rice-eating crabs in rice paddies, splashing juices containing metacercariae, can also transmit the parasite, or using juices strained from fresh crabs for medicinal uses. This parasite is easily spread because it is able to infect other animals (zoonosis). An assortment of mammals and birds can be infected and act as paratenic hosts. Ingestion of the paratenic host can lead to infection of this parasite. | |||
''Paragonimus westermani'' is distributed in southeast Asia and Japan. Other species of Paragonimus are common in parts of Asia, Africa and South and Central America. ''P. westermani'' has been increasingly recognized in the United States during the past 15 years because of the increase of immigrants from endemic areas such as Southeast Asia. Estimated to infect 22 million people worldwide.<ref name=CDCpage2 /> | |||
== Transmission == | |||
Transmission of the parasite ''P. westermani'' to humans and mammals primarily occurs through the consumption of raw or undercooked seafood. In Asia, an estimated 80% of freshwater crabs carry ''P. westermani''.<ref name= Pachucki1984 /> In preparation, live crabs are crushed and metacercariae may contaminate the fingers/utensils of the person preparing the meal. Accidental transfer of infective cysts can occur via food preparers who handle raw seafood and subsequently contaminate cooking utensils and other foods.<ref name= Yokogawa1965 /> Consumption of animals which feed on crustaceans can also transmit the parasite, for cases have been cited in Japan where raw boar meat was the source of human infection.<ref name=Markell2006 /><ref name=Miyazaki1976 /> | |||
Food preparation techniques such as pickling and salting do not exterminate the causative agent. For example, in a Chinese study eating "drunken crabs" was shown to be particularly risky because the infection rate was 100% when crabs are immersed in wine for 3–5 minutes and fed to cats/dog.<ref name=Markell2006 /> | |||
== Reservoir == | |||
Animals such as pigs, dogs, and a variety of feline species can also harbor ''P. westermani''.<ref name=CDCpage2 /> | |||
== Vector == | |||
There is no vector, but various snail and crab species serve as intermediate hosts. In Japan and Korea, the crab species ''[[Eriocheir]]'' is an important item of food as well as a notable second intermediate host of the parasite.<ref name=Markell2006 /> | |||
== Incubation period == | |||
Time from infection to oviposition (laying eggs) is 65 to 90 days. | |||
Infections may persist for 20 years in humans.<ref name=CDCpage2 /> | |||
== Pathology == | |||
Once in the lung or ectopic site, the worm stimulates an inflammatory response that allows it to cover itself in granulation tissue forming a capsule. These capsules can ulcerate and heal over time. The eggs in the surrounding tissue become pseudotubercles. If the worm becomes disseminated and gets into the spinal cord, it can cause paralysis; capsules in the heart can cause death. The symptoms are localized in the pulmonary system, which include a bad cough, bronchitis, and blood in sputum (hemoptysis). | |||
== Diagnosis == | |||
Diagnosis is based on microscopic demonstration of eggs in stool or sputum, but these are not present until 2 to 3 months after infection. However, eggs are also occasionally encountered in effusion fluid or biopsy material. Furthermore, you can use morphologic comparisons with other intestinal parasites to diagnose potential causative agents. Finally, antibody detection is useful in light infections and in the diagnosis of extrapulmonary paragonimiasis. In the United States, detection of antibodies to Paragonimus westermani has helped physicians differentiate paragonimiasis from tuberculosis in Indochinese immigrants.<ref name=CDCpage2 /> | |||
Additionally, radiological methods can be used to X-ray the chest cavity and look for worms. This method is easily misdiagnosed, because pulmonary infections look like tuberculosis, pneumonia, or spirochaetosis. A lung biopsy can also be used to diagnose this parasite. | |||
== Management and treatment == | |||
According to the CDC, [[praziquantel]] is the drug of choice to treat paragonimiasis.<ref name=CDCpage2 /> The recommended dosage of 75 mg/kg per day, divided into 3 doses over 3 days has proven to eliminate P. westermani.<ref name= Pachucki1984 /> [[Bithionol]] is an alternative drug for treatment of this disease but is associated with skin rashes and urticaria. For additional information, see the recommendations in The Medical Letter (Drugs for Parasitic Infections). | |||
== Clinical presentation in humans == | |||
Case study:<ref name=Heath1997 /> <blockquote> | |||
An 11½-year-old Hmong Laotian boy was brought into the emergency room by his parents with a 2- to 3-month history of decreasing stamina and increasing dyspnea [shortness of breath] on exertion. He described an intermittent nonproductive cough and decreased appetite and was thought to have lost weight. He denied fever, chills, night sweats, headache, palpitations, hemoptysis [coughing up blood], chest pain, vomiting, diarrhea or urticaria [skin rash notable for dark red, raised, itchy bumps]. There were no pets at home. At the time of immigration to the United States 16 months earlier, all family members had negative purified protein derivative intradermal tests except one brother, who was positive but had a normal chest radiograph and subsequently received isoniazid for 12 months… a left lateral thoracotomy was performed during which 1800 ml of an odorless, cloudy, pea soup-like fluid containing a pale yellow, cottage cheese-like, proteinaceous material was removed, along with a solitary, 6-mm-long, reddish brown fluke subsequently identified as Paragonimus westermani | |||
</blockquote> | |||
Human infection with Paragonimus may cause acute or chronic symptoms, and manifestations may be either pulmonary or extrapulmonary.<ref name=Chung1981 /> | |||
Acute symptoms: | |||
The acute phase (invasion and migration) may be marked by diarrhea, abdominal pain, fever, cough, urticaria, hepatosplenomegaly, pulmonary abnormalities, and eosinophilia.<ref name=CDCpage2 /> The acute stage corresponds to the period of invasion and migration of flukes and consists of abdominal pain, diarrhea and urticaria, followed roughly 1 to 2 weeks later by fever, pleuritic chest pain, cough and/or dyspnea.<ref name=Heath1997 /> | |||
Chronic Symptoms: | |||
During the chronic phase, pulmonary manifestations include cough, expectoration of discolored sputum, hemoptysis, and chest radiographic abnormalities.<ref name=CDCpage2 /> Chronic pulmonary paragonimiasis, the most common clinical pattern, is frequently mild, with chronic cough, brown-tinged sputum (the color being caused by expectorated clusters of reddish brown eggs rather than by blood) and true hemoptysis.<ref name=Heath1997 /> | |||
== Confusion with Tuberculosis == | |||
Practitioners should always consider the possibility of tuberculosis in patients with fevers, cough, weight loss. However, in endemic areas it is prudent to consider paragonimiasis as well. Flukes occasionally cause confusion when they invade the pleural space without entering the lung [[parenchyma]].<ref name=Roberts1988 /><ref name=Minh1981 /><ref name=Johnson1982 /> | |||
<blockquote> | |||
“In contrast to tuberculosis, pulmonary paragonimiasis is only rarely accompanied by rales or other adventitious breath sounds. Many patients are asymptomatic, and symptomatic patients frequently look well despite a prolonged course.” | |||
</blockquote> | |||
In pleural paragonimiasis, symptoms may be minimal and diagnosis complicated, since ova are not coughed or spit out or swallowed and there is frequently no cough. Such patients may develop pleural effusions and, because of the coendemicity with Mycobacterium tuberculosis (and co-infection in some patients), such effusions are often misdiagnosed as isolated tuberculosis.<ref name=Johnson1983 /><ref name=Romeo1986 /> | |||
*Adapted from Heath, Harley W & Susan G Marshall. "Pleural Paragonimiasis In A Laotian Child.* | |||
Extra-pulmonary locations of the adult worms result in more severe manifestations, especially when the brain is involved. Extra-pulmonary paragonimiasis is rarely seen in humans, as the worms nearly exclusively migrate to the lungs. Despite this, cysts can develop in the brain and abdominal adhesions resulting from infection have been reported. Cysts may contain living or dead worms; a yellow-brownish thick fluid (occasionally hemorrhagic). When the worm dies or escapes, the cysts gradually shrink, leaving nodules of fibrous tissues and eggs which can calcify.<ref name=Markell2006 /> | |||
Worldwide the most common cause of [[hemoptysis]] is paragonimiasis.<ref name=Davis1999 /> | |||
Other case studies: | |||
*{{cite journal | author = Pachucki CT, Levandowski RA, Brown VA, Sonnenkalb BH, Vruno MJ | year = 1984 | title = American paragonimiasis treated with Praziquantel | url = | journal = New Eng J Med | volume = 311 | issue = | pages = 582–3 | doi=10.1056/nejm198408303110906}} | |||
*{{cite journal | author = Procop GW, Marty AM, Scheck DN, Mease DR, Maw GM | year = 2000 | title = North American Paragonimiasis: A case report | url = | journal = Acta Cytol | volume = 44 | issue = | pages = 75–80 | doi=10.1159/000326230}} | |||
== Public health and prevention strategies == | |||
Prevention programs should promote more hygienic food preparation by encouraging safer cooking techniques and more sanitary handling of potentially contaminated seafood. The elimination of the first intermediate host, the snail, is not tenable due to the nature of the organisms habits.<ref name=Markell2006 /> A key component to prevention is research, more specifically the research of everyday behaviors. This recent study was conducted as a part of a broader effort to determine the status of Paragonimus species infection in Laos.<ref name=Song2008 /> An epidemiological survey was conducted on villagers and schoolchildren in Namback District between 2003 and 2005. Among 308 villagers and 633 primary and secondary schoolchildren, 156 villagers and 92 children had a positive reaction on a Paragonimus skin test. Consequently, several types of crabs were collected from markets and streams in a paragonimiasis endemic area for the inspection of metacercariae and were identified as the second intermediate host of the Paragonimus species. In this case study, we see how high prevalence of paragonimiasis is explained by dietary habits of the population. Amongst schoolchildren, many students reported numerous experiences of eating roast crabs in the field. Adult villagers reported frequent consumption of seasoned crabs (Tan Cheoy Koung) and papaya salad (Tammack Koung) with crushed raw crab. In addition to this characteristic feature of the villagers' food culture, the denizens of this area drink fresh crab juice as a traditional cure for measles, and this was also thought to constitute a route for infection. | |||
== See also == | |||
*[[List of parasites (human)]] | |||
== References == | |||
This article incorporates CC-BY-3.0 text from the reference.<ref name="Appleton 2009">Appleton C. C., Forbes A. T.& Demetriades N. T. (2009). "The occurrence, bionomics and potential impacts of the invasive freshwater snail ''Tarebia granifera'' (Lamarck, 1822) (Gastropoda: Thiaridae) in South Africa". ''Zoologische Mededelingen'' '''83'''. http://www.zoologischemededelingen.nl/83/nr03/a04</ref> | |||
{{reflist |colwidth=30em |refs= | |||
<ref name=Manson1881>{{vcite journal |author=Manson, P |year=1881 |title=Distoma ringeri |journal=Med. Times Gaz. |volume=2 |pages=8–9 |url=http://cmr.asm.org/cgi/content/full/15/4/595#R175}}</ref> | |||
<ref name=Muller1996>{{vcite book |author=Muller, R |year=1996 |chapter=Liver and lung flukes |pages=274–285 |editor= Cox FEG |title=The Wellcome Trust illustrated history of tropical diseases |publisher=The Wellcome Trust, London, United Kingdom |url=http://cmr.asm.org/cgi/content/full/15/4/595#R193}}</ref> | |||
<ref name=Grove1990>{{vcite book |author=Grove, DI |year=1990 |title=A history of human helminthology |publisher=CAB International, Wallingford, United Kingdom |url= http://cmr.asm.org/cgi/content/full/15/4/595#R105}}</ref> | |||
<ref name=Desowitz1987>{{vcite book |author=Desowitz, R |title=New Guinea Tapeworms and Jewish Grandmothers: Tales of Parasites and People |publisher=New York: WW Norton |year=1987 |isbn=978-0-393-30426-8}}</ref> | |||
<ref name=Chung1981>{{vcite journal |author=Chung HL, Ho LY, Hsu CP, Ts'ao WJ |title=Recent progress in studies of Paragonimus and paragonimiasis control in China |journal=Chin Med J |year=1981 |volume=94 |pages=483–494}}</ref> | |||
<ref name=CDCpage2>{{vcite web |url=http://www.dpd.cdc.gov/DPDx/html/Frames/MR/Paragonimiasis/body_Paragonimiasis_page2.htm |title=CDC Paragonimiasis}}</ref> | |||
<ref name=Heath1997>{{vcite journal |author=Heath HW, Marshall SG |title= Pleural Paragonimiasis In A Laotian Child |journal= Pediatric Infectious Disease Journal |volume= 16 | issue = 12 |year=1997 |pages=1182–1185 |url=http://www.pidj.com/pt/re/pidj/abstract.00006454-199712000-00018.htm;jsessionid=JnzNJ8ynh33JPL3GQ1NvLlxh6MJTQMkC7SscXNvPj6p6Yd5QfyZ4!-858031623!181195628!8091!-1 |doi=10.1097/00006454-199712000-00018}}</ref> | |||
<ref name=Roberts1988>{{vcite journal |author=Roberts PP |title= Parasitic infections of the pleural space |journal=Semin Respir Infect |year=1988 |volume=3 |pages=362–382}}</ref> | |||
<ref name=Minh1981>{{vcite journal |author=Minh VD, Engle P, Greenwood JR, Prendergast TJ, Salness K, St. Clair R |title=Pleural paragonimiasis in a Southeast Asian refugee |journal=Am Rev Respir Dis |year=1981 |volume=124 |pages=186–188}}</ref> | |||
<ref name=Johnson1982>{{vcite journal |author=Johnson JR, Falk A, Iber C, Davies S |title= Paragonimiasis in the United States: a report of nine cases in Hmong immigrants |journal= Chest |year=1982 |volume=82 |pages=168–171 | pmid=7094646 | doi=10.1378/chest.82.2.168 }}</ref> | |||
<ref name=Johnson1983>{{vcite journal |author=Johnson RJ, Johnson JR |title=Paragonimiasis in Indochinese refugees: roentgenographic findings and clinical correlations |journal=Am Rev Respir Dis |year=1983 |volume=128 |pages=534–538}}</ref> | |||
<ref name=Romeo1986>{{vcite journal |author=Romeo DP, Pollock JJ |title= Pulmonary paragonimiasis: diagnostic value of pleural fluid analysis |journal=South Med J |year= 1986 |volume=79 |pages=241–243 | pmid=3945854 |doi=10.1097/00007611-198602000-00024}}</ref> | |||
<ref name=Davis1999>{{vcite book |author=Davis, GS, Elizabeth AS |editor= Marcy TW |title= Medical Management of Pulmonary Diseases |publisher=CRC Press |year=1999 |page=345}}</ref> | |||
<ref name=Markell2006>{{vcite book |title=Markell and Voge's Medical Parasitology 9th Edition |year=2006 |page= 200 |isbn=978-0-7216-4793-7}}</ref> | |||
<ref name=Miyazaki1976>{{vcite journal |author=Miyazaki I, Habe S |title= A newly recognized mode of human infection with the lung fluke, Paragonimus westermani. |journal=J Parasitol |year=1976 |volume=62 |pages=646–8 |doi=10.2307/3279438}}</ref> | |||
<ref name= Pachucki1984>{{vcite journal | author=Pachucki, CT, Levandowski, RA, Brown, VA, Sonnenkalb, BH, Vruno, MJ |title= American Paragonimiasis treated with praziquantel |journal= New Eng J Med |year=1984 |volume= 311 |pages=582–583 |doi=10.1056/nejm198408303110906}}</ref> | |||
<ref name= Yokogawa1965>{{vcite journal |author= Yokogawa, M |title= Paragonimus and Paragonimiasis |journal= Adv Parasitol |year=1965 |volume=3 |pages=99–158 |doi=10.1016/s0065-308x(08)60364-4}}</ref> | |||
= | <ref name=Song2008>{{vcite journal |author= Song HO, Min DY, Rim HJ, Youthanavanh V, Dalunyi B, Sengdara V, Virasack B, Bounlay P. |title=Skin Test for Paragonimiasis among Schoolchildren and Villagers in Namback District, Luangprabang Province, Lao PDR. |journal=The Korean Journal of Parasitology |year= 2008 |volume= 46|issue=3 |pages=179–82 | doi = 10.3347/kjp.2008.46.3.179 }}</ref> | ||
}} | }} | ||
{{ | ==Further reading== | ||
* {{cite book |title=Markell and Voge's Medical Parasitology |edition=9th |pages=198, 201 }} | |||
* {{cite web |publisher=CDC |url=http://www.dpd.cdc.gov/DPDx/html/Paragonimiasis.htm |title=Paragonimiasis }} | |||
* {{cite book |author=Muller, R |year=1996|contribution=Liver and lung flukes|pages=274-285|editor=F. E. G. Cox |title=The Wellcome Trust illustrated history of tropical diseases|publisher=The Wellcome Trust |location=London |url=http://cmr.asm.org/cgi/content/full/15/4/595#R193 }} | |||
* {{vcite journal | author = Heath Harley W, Marshall Susan G | year = 1997 | title = Pleural Paragonimiasis In A Laotian Child | url = http://www.pidj.com/pt/re/pidj/abstract.00006454-199712000-00018.htm | journal = Pediatric Infectious Disease Journal | volume = 16 | issue = 12| pages = 1182–1185 | doi=10.1097/00006454-199712000-00018}} | |||
* {{vcite journal | author = Yokogawa M | year = 1965 | title = Paragonimus and Paragonimiasis | journal = [[Adv Parasitol]] | volume = 3 | issue = | pages = 99–158 | doi=10.1016/s0065-308x(08)60364-4}} | |||
* {{vcite journal | author = Pachucki CT, Levandowski RA, Brown VA, Sonnenkalb BH, Vruno MJ | year = 1984 | title = American Paragonimiasis treated with praziquantel | journal = New Eng J Med | volume = 311 | issue = | pages = 582–583 | doi=10.1056/nejm198408303110906}} | |||
* {{cite book |title=Foundations of Parasitology|authors=Larry S. Roberts, John Janovy Jr.|edition=7th|publisher=McGraw Hill |year=2005 |pages=279–283 }} | |||
* {{vcite journal |url=wwwnc.cdc.gov/eid/article/3/3/pdfs/97-0306.pdf |title=Emerging and Reemerging Helminthiases and the Public Health of China |authors=Peter J. Hotez, Feng Zheng, Xu Long-qi, Chen Ming-gang, Xiao Shu-hua, Liu Shu-xian, David Blair, Donald P. McManus, George M. Davis |publisher=CDC |journal=[[Emerging Infectious Diseases]] |volume=3 |issue=3 |date=July–September 1997 |pages=303-310 }} | |||
== External links == | |||
* [http://www.dpd.cdc.gov/DPDx/html/Paragonimiasis.htm Center for Disease Control paragonimiasis web article] | |||
* [http://www.path.cam.ac.uk/~schisto/OtherFlukes/Paragonimus.html The Human Lung Fluke - Paragonimus westermani] at Cambridge Schistosomiasis Research Group | |||
{{Helminthiases}} | |||
{{ | {{DEFAULTSORT:Paragonimus Westermani}} | ||
[[Category:Digenea]] | |||
[[Category:Parasitic animals]] | |||
Latest revision as of 16:00, 6 August 2015
|
Paragonimus infection Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Paragonimus westermani On the Web |
|
American Roentgen Ray Society Images of Paragonimus westermani |
|
Risk calculators and risk factors for Paragonimus westermani |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Paragonimus westermani is the major species of lung fluke to infects humans, causing paragonimiasis. The species sometimes is called the Japanese Lung fluke or Oriental Lung fluke. Human infections are most common in eastern Asia and in South America. Paragonimus westermani was discovered when two Bengal tigers died of paragonimiasis in zoos in Europe in 1878. Several years later Infections in humans were recognised in Formosa.
Introduction
Paragonimiasis is a food-borne parasitic infection caused by the lung fluke. It may cause a sub-acute to chronic inflammatory disease of the lung. It is one of the most familiar lung flukes with the widest geographical range. It was discovered by Coenraad Kerbert (1849-1927) in 1878.
Causative agent
More than 30 species of trematodes (flukes) of the genus Paragonimus have been reported to infect animals and humans. Among the more than 10 species reported to infect humans, the commonest is Paragonimus westermani, the oriental lung fluke.[1][2]
Morphology
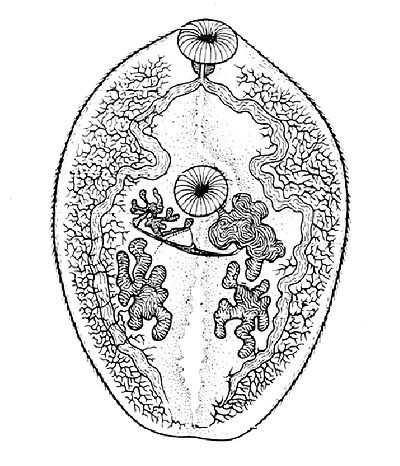
In size, shape, and color, Paragonimus westermani resembles a coffee bean when alive. Adult worms are 7.5 mm to 12 mm long and 4 mm to 6 mm wide. The thickness ranges from 3.5 mm to 5 mm. The skin of the worm (tegument) is thickly covered with scalelike spines. The oral and ventral suckers are similar in size, with the latter placed slightly pre-equatorially. The excretory bladder extends from the posterior end to the pharynx. The lobed testes are adjacent from each other located at the posterior end, and the lobed ovaries are off-centered near the center of the worm (slightly postacetabular). The uterus is located in a tight coil to the right of the acetabulum, which is connected, to the vas deferens. The vitelline glands, which produce the yolk for the eggs, are widespread in the lateral field from the pharynx to the posterior end. Inspection of the tegumental spines and shape of the metacercariae may distinguish between the 30-odd species of Paragonimus spp. but the distinction is sufficiently difficult to justify suspicion that many of the described species are synonyms.[3]
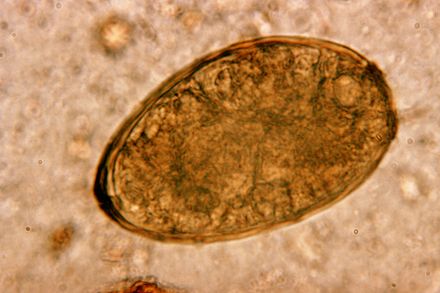
- Eggs: Paragonimus westermani eggs range from 80 to 120 µm long by 45 to 70 µm wide. They are yellow-brown, ovoid or elongate, with a thick shell, and often asymmetrical with one end slightly flattened. At the large end, the operculum is clearly visible. The opposite (abopercular) end is thickened. The eggs are unembryonated when passed in sputum or feces.[2]
- Cercaria (not shown): Cercariae are often indistinguishable between species. There is a large posterior sucker, and the exterior is spined.
- Metacercaria: Metacercariae are usually encysted in tissue. The exterior is spined and has two suckers
- Adults: Adult flukes are typically reddish brown and ovoid, measuring 7 to 16 mm by 4 to 8 mm, similar in size and appearance to a coffee bean.They are hermaphroditic, with a lobed ovary located anterior to two branching testes. Like all members of the Trematoda, they possess oral and ventral suckers.
History of discovery
P. westermani was discovered in the lungs of a human by Ringer in 1879[4] and eggs in the sputum were recognized independently by Manson and Erwin von Baelz in 1880.[4][5] Manson proposed the snail as an intermediate host and various Japanese workers detailed the whole life cycle in the snail between 1916 and 1922.[6] The species name P. westermani was named after Pieter Westerman (1859-1925) a zookeeper who noted the trematode in a Bengal tiger in an Amsterdam Zoo.[7]
Life cycle
Unembryonated eggs are passed in the sputum of a human or feline. Two weeks later, miracidia develop in the egg and hatches. The miracidia penetrate its first intermediate host (snail). Within the snail mother sporocyst form and produce many mother rediae, which subsequently produce many daughter rediae which shed crawling cercariae into fresh water. The crawling cercariae penetrate fresh water crabs and encyst in its muscles becoming metacercaria. Humans or felines then eat the infected crabs raw. Once eaten, the metacercaria excysts and penetrates the gut, diaphragm and lung where it becomes an adult worm in pairs.
The first intermediate hosts of the Paragonimus westermani are freshwater snails:
- Semisulcospira amurensis[8]
- Semisulcospira calculus[8]
- Semisulcospira cancellata[8]
- Semisulcospira extensa[8]
- Semisulcospira gottschei[8]
- Semisulcospira libertina - synonym: Semisulcospira toucheana[8]
- Semisulcospira mandarina - synonym: Semisulcospira wegckiangensis[8]
- Semisulcospira multicincta[8]
- Semisulcospira nodiperda[8]
- Semisulcospira nodiperda quinaria[8]
- Semisulcospira paucincta[8]
- Semisulcospira peregrinomum[8]
For many years Tarebia granifera was believed[8] to be an intermediate host for the Paragonimus westermani, but Michelson showed in 1992 that this was erroneous.[9][10]
Paragonimus has a quite complex life-cycle that involves two intermediate hosts as well as humans. Eggs first develop in water after being expelled by coughing (unembryonated) or being passed in human feces. In the external environment, the eggs become embryonated. In the next stage, the parasite miracidia hatch and invades the first intermediate host such as a species of freshwater snail. Miracidia penetrate its soft tissues and go through several developmental stages inside the snail but mature into cercariae in 3 to 5 months. Cercariae next invade the second intermediate host such as crabs or crayfish and encyst to develop into metacercariae within 2 months. Infection of humans or other mammals (definitive hosts) occurs via consumption of raw or undercooked crustaceans. Human infection with P. westermani occurs by eating inadequately cooked or pickled crab or crayfish that harbor metacercariae of the parasite. The metacercariae excyst in the duodenum, penetrate through the intestinal wall into the peritoneal cavity, then through the abdominal wall and diaphragm into the lungs, where they become encapsulated and develop into adults. The worms can also reach other organs and tissues, such as the brain and striated muscles, respectively. However, when this takes place completion of the life cycles is not achieved, because the eggs laid cannot exit these sites.[2]
Epidemiology
Reservoir hosts of Paragonimus spp. include numerous species of carnivores including felids, canids, viverrids, mustelids, some rodents and pigs. Humans become infected after eating raw freshwater crabs or crayfish that have been encysted with the metacerciaria. Southeast Asia is more predominately more infected because of lifestyles. Raw seafood is popular in these countries. Crab collectors string raw crabs together and bring them miles inland to sell in Taiwan markets. These raw crabs are then marinated or pickled in vinegar or wine to coagulate the crustacean muscle. This method of preparation does not kill the metacercariae, consequently infecting the host. Smashing rice-eating crabs in rice paddies, splashing juices containing metacercariae, can also transmit the parasite, or using juices strained from fresh crabs for medicinal uses. This parasite is easily spread because it is able to infect other animals (zoonosis). An assortment of mammals and birds can be infected and act as paratenic hosts. Ingestion of the paratenic host can lead to infection of this parasite.
Paragonimus westermani is distributed in southeast Asia and Japan. Other species of Paragonimus are common in parts of Asia, Africa and South and Central America. P. westermani has been increasingly recognized in the United States during the past 15 years because of the increase of immigrants from endemic areas such as Southeast Asia. Estimated to infect 22 million people worldwide.[2]
Transmission
Transmission of the parasite P. westermani to humans and mammals primarily occurs through the consumption of raw or undercooked seafood. In Asia, an estimated 80% of freshwater crabs carry P. westermani.[11] In preparation, live crabs are crushed and metacercariae may contaminate the fingers/utensils of the person preparing the meal. Accidental transfer of infective cysts can occur via food preparers who handle raw seafood and subsequently contaminate cooking utensils and other foods.[12] Consumption of animals which feed on crustaceans can also transmit the parasite, for cases have been cited in Japan where raw boar meat was the source of human infection.[1][13] Food preparation techniques such as pickling and salting do not exterminate the causative agent. For example, in a Chinese study eating "drunken crabs" was shown to be particularly risky because the infection rate was 100% when crabs are immersed in wine for 3–5 minutes and fed to cats/dog.[1]
Reservoir
Animals such as pigs, dogs, and a variety of feline species can also harbor P. westermani.[2]
Vector
There is no vector, but various snail and crab species serve as intermediate hosts. In Japan and Korea, the crab species Eriocheir is an important item of food as well as a notable second intermediate host of the parasite.[1]
Incubation period
Time from infection to oviposition (laying eggs) is 65 to 90 days. Infections may persist for 20 years in humans.[2]
Pathology
Once in the lung or ectopic site, the worm stimulates an inflammatory response that allows it to cover itself in granulation tissue forming a capsule. These capsules can ulcerate and heal over time. The eggs in the surrounding tissue become pseudotubercles. If the worm becomes disseminated and gets into the spinal cord, it can cause paralysis; capsules in the heart can cause death. The symptoms are localized in the pulmonary system, which include a bad cough, bronchitis, and blood in sputum (hemoptysis).
Diagnosis
Diagnosis is based on microscopic demonstration of eggs in stool or sputum, but these are not present until 2 to 3 months after infection. However, eggs are also occasionally encountered in effusion fluid or biopsy material. Furthermore, you can use morphologic comparisons with other intestinal parasites to diagnose potential causative agents. Finally, antibody detection is useful in light infections and in the diagnosis of extrapulmonary paragonimiasis. In the United States, detection of antibodies to Paragonimus westermani has helped physicians differentiate paragonimiasis from tuberculosis in Indochinese immigrants.[2]
Additionally, radiological methods can be used to X-ray the chest cavity and look for worms. This method is easily misdiagnosed, because pulmonary infections look like tuberculosis, pneumonia, or spirochaetosis. A lung biopsy can also be used to diagnose this parasite.
Management and treatment
According to the CDC, praziquantel is the drug of choice to treat paragonimiasis.[2] The recommended dosage of 75 mg/kg per day, divided into 3 doses over 3 days has proven to eliminate P. westermani.[11] Bithionol is an alternative drug for treatment of this disease but is associated with skin rashes and urticaria. For additional information, see the recommendations in The Medical Letter (Drugs for Parasitic Infections).
Clinical presentation in humans
Case study:[14]
An 11½-year-old Hmong Laotian boy was brought into the emergency room by his parents with a 2- to 3-month history of decreasing stamina and increasing dyspnea [shortness of breath] on exertion. He described an intermittent nonproductive cough and decreased appetite and was thought to have lost weight. He denied fever, chills, night sweats, headache, palpitations, hemoptysis [coughing up blood], chest pain, vomiting, diarrhea or urticaria [skin rash notable for dark red, raised, itchy bumps]. There were no pets at home. At the time of immigration to the United States 16 months earlier, all family members had negative purified protein derivative intradermal tests except one brother, who was positive but had a normal chest radiograph and subsequently received isoniazid for 12 months… a left lateral thoracotomy was performed during which 1800 ml of an odorless, cloudy, pea soup-like fluid containing a pale yellow, cottage cheese-like, proteinaceous material was removed, along with a solitary, 6-mm-long, reddish brown fluke subsequently identified as Paragonimus westermani
Human infection with Paragonimus may cause acute or chronic symptoms, and manifestations may be either pulmonary or extrapulmonary.[15]
Acute symptoms: The acute phase (invasion and migration) may be marked by diarrhea, abdominal pain, fever, cough, urticaria, hepatosplenomegaly, pulmonary abnormalities, and eosinophilia.[2] The acute stage corresponds to the period of invasion and migration of flukes and consists of abdominal pain, diarrhea and urticaria, followed roughly 1 to 2 weeks later by fever, pleuritic chest pain, cough and/or dyspnea.[14] Chronic Symptoms: During the chronic phase, pulmonary manifestations include cough, expectoration of discolored sputum, hemoptysis, and chest radiographic abnormalities.[2] Chronic pulmonary paragonimiasis, the most common clinical pattern, is frequently mild, with chronic cough, brown-tinged sputum (the color being caused by expectorated clusters of reddish brown eggs rather than by blood) and true hemoptysis.[14]
Confusion with Tuberculosis
Practitioners should always consider the possibility of tuberculosis in patients with fevers, cough, weight loss. However, in endemic areas it is prudent to consider paragonimiasis as well. Flukes occasionally cause confusion when they invade the pleural space without entering the lung parenchyma.[16][17][18]
“In contrast to tuberculosis, pulmonary paragonimiasis is only rarely accompanied by rales or other adventitious breath sounds. Many patients are asymptomatic, and symptomatic patients frequently look well despite a prolonged course.”
In pleural paragonimiasis, symptoms may be minimal and diagnosis complicated, since ova are not coughed or spit out or swallowed and there is frequently no cough. Such patients may develop pleural effusions and, because of the coendemicity with Mycobacterium tuberculosis (and co-infection in some patients), such effusions are often misdiagnosed as isolated tuberculosis.[19][20]
- Adapted from Heath, Harley W & Susan G Marshall. "Pleural Paragonimiasis In A Laotian Child.*
Extra-pulmonary locations of the adult worms result in more severe manifestations, especially when the brain is involved. Extra-pulmonary paragonimiasis is rarely seen in humans, as the worms nearly exclusively migrate to the lungs. Despite this, cysts can develop in the brain and abdominal adhesions resulting from infection have been reported. Cysts may contain living or dead worms; a yellow-brownish thick fluid (occasionally hemorrhagic). When the worm dies or escapes, the cysts gradually shrink, leaving nodules of fibrous tissues and eggs which can calcify.[1]
Worldwide the most common cause of hemoptysis is paragonimiasis.[21]
Other case studies:
- Pachucki CT, Levandowski RA, Brown VA, Sonnenkalb BH, Vruno MJ (1984). "American paragonimiasis treated with Praziquantel". New Eng J Med. 311: 582–3. doi:10.1056/nejm198408303110906.
- Procop GW, Marty AM, Scheck DN, Mease DR, Maw GM (2000). "North American Paragonimiasis: A case report". Acta Cytol. 44: 75–80. doi:10.1159/000326230.
Public health and prevention strategies
Prevention programs should promote more hygienic food preparation by encouraging safer cooking techniques and more sanitary handling of potentially contaminated seafood. The elimination of the first intermediate host, the snail, is not tenable due to the nature of the organisms habits.[1] A key component to prevention is research, more specifically the research of everyday behaviors. This recent study was conducted as a part of a broader effort to determine the status of Paragonimus species infection in Laos.[22] An epidemiological survey was conducted on villagers and schoolchildren in Namback District between 2003 and 2005. Among 308 villagers and 633 primary and secondary schoolchildren, 156 villagers and 92 children had a positive reaction on a Paragonimus skin test. Consequently, several types of crabs were collected from markets and streams in a paragonimiasis endemic area for the inspection of metacercariae and were identified as the second intermediate host of the Paragonimus species. In this case study, we see how high prevalence of paragonimiasis is explained by dietary habits of the population. Amongst schoolchildren, many students reported numerous experiences of eating roast crabs in the field. Adult villagers reported frequent consumption of seasoned crabs (Tan Cheoy Koung) and papaya salad (Tammack Koung) with crushed raw crab. In addition to this characteristic feature of the villagers' food culture, the denizens of this area drink fresh crab juice as a traditional cure for measles, and this was also thought to constitute a route for infection.
See also
References
This article incorporates CC-BY-3.0 text from the reference.[10]
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Markell and Voge's Medical Parasitology 9th Edition. 2006. ISBN 978-0-7216-4793-7. p. 200.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 2.9 CDC Paragonimiasis.
- ↑ Janovy, John; Schmidt, Gerald D.; Roberts, Larry S. (1996). Gerald D. Schmidt & Larry S. Roberts' Foundations of parasitology. Dubuque, Iowa: Wm. C. Brown. ISBN 0-697-26071-2.
- ↑ 4.0 4.1 Muller, R. Liver and lung flukes. In: Cox FEG. The Wellcome Trust illustrated history of tropical diseases. The Wellcome Trust, London, United Kingdom; 1996. p. 274–285.
- ↑ Manson, P. Distoma ringeri. Med. Times Gaz.. 1881;2:8–9.
- ↑ Grove, DI. A history of human helminthology. CAB International, Wallingford, United Kingdom; 1990.
- ↑ Desowitz, R. New Guinea Tapeworms and Jewish Grandmothers: Tales of Parasites and People. New York: WW Norton; 1987. ISBN 978-0-393-30426-8.
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 8.11 8.12 World Health Organization (1995). Control of Foodborne Trematode Infection. WHO Technical Report Series. 849. PDF part 1, PDF part 2. page 125-126.
- ↑ Michelson E (1992). "Thiara granifera: a victim of authoritarianism?". Malacological Review. 25: 67–71.
- ↑ 10.0 10.1 Appleton C. C., Forbes A. T.& Demetriades N. T. (2009). "The occurrence, bionomics and potential impacts of the invasive freshwater snail Tarebia granifera (Lamarck, 1822) (Gastropoda: Thiaridae) in South Africa". Zoologische Mededelingen 83. http://www.zoologischemededelingen.nl/83/nr03/a04
- ↑ 11.0 11.1 Pachucki, CT, Levandowski, RA, Brown, VA, Sonnenkalb, BH, Vruno, MJ. American Paragonimiasis treated with praziquantel. New Eng J Med. 1984;311:582–583. doi:10.1056/nejm198408303110906.
- ↑ Yokogawa, M. Paragonimus and Paragonimiasis. Adv Parasitol. 1965;3:99–158. doi:10.1016/s0065-308x(08)60364-4.
- ↑ Miyazaki I, Habe S. A newly recognized mode of human infection with the lung fluke, Paragonimus westermani.. J Parasitol. 1976;62:646–8. doi:10.2307/3279438.
- ↑ 14.0 14.1 14.2 Heath HW, Marshall SG. Pleural Paragonimiasis In A Laotian Child. Pediatric Infectious Disease Journal. 1997;16(12):1182–1185. doi:10.1097/00006454-199712000-00018.
- ↑ Chung HL, Ho LY, Hsu CP, Ts'ao WJ. Recent progress in studies of Paragonimus and paragonimiasis control in China. Chin Med J. 1981;94:483–494.
- ↑ Roberts PP. Parasitic infections of the pleural space. Semin Respir Infect. 1988;3:362–382.
- ↑ Minh VD, Engle P, Greenwood JR, Prendergast TJ, Salness K, St. Clair R. Pleural paragonimiasis in a Southeast Asian refugee. Am Rev Respir Dis. 1981;124:186–188.
- ↑ Johnson JR, Falk A, Iber C, Davies S. Paragonimiasis in the United States: a report of nine cases in Hmong immigrants. Chest. 1982;82:168–171. doi:10.1378/chest.82.2.168. PMID 7094646.
- ↑ Johnson RJ, Johnson JR. Paragonimiasis in Indochinese refugees: roentgenographic findings and clinical correlations. Am Rev Respir Dis. 1983;128:534–538.
- ↑ Romeo DP, Pollock JJ. Pulmonary paragonimiasis: diagnostic value of pleural fluid analysis. South Med J. 1986;79:241–243. doi:10.1097/00007611-198602000-00024. PMID 3945854.
- ↑ Davis, GS, Elizabeth AS. In: Marcy TW. Medical Management of Pulmonary Diseases. CRC Press; 1999. p. 345.
- ↑ Song HO, Min DY, Rim HJ, Youthanavanh V, Dalunyi B, Sengdara V, Virasack B, Bounlay P.. Skin Test for Paragonimiasis among Schoolchildren and Villagers in Namback District, Luangprabang Province, Lao PDR.. The Korean Journal of Parasitology. 2008;46(3):179–82. doi:10.3347/kjp.2008.46.3.179.
Further reading
- Markell and Voge's Medical Parasitology (9th ed.). pp. 198, 201.
- "Paragonimiasis". CDC.
- Muller, R (1996). "Liver and lung flukes". In F. E. G. Cox. The Wellcome Trust illustrated history of tropical diseases. London: The Wellcome Trust. pp. 274–285.
- Heath Harley W, Marshall Susan G. Pleural Paragonimiasis In A Laotian Child. Pediatric Infectious Disease Journal. 1997;16(12):1182–1185. doi:10.1097/00006454-199712000-00018.
- Yokogawa M. Paragonimus and Paragonimiasis. Adv Parasitol. 1965;3:99–158. doi:10.1016/s0065-308x(08)60364-4.
- Pachucki CT, Levandowski RA, Brown VA, Sonnenkalb BH, Vruno MJ. American Paragonimiasis treated with praziquantel. New Eng J Med. 1984;311:582–583. doi:10.1056/nejm198408303110906.
- Larry S. Roberts, John Janovy Jr. (2005). Foundations of Parasitology (7th ed.). McGraw Hill. pp. 279–283.
- Peter J. Hotez, Feng Zheng, Xu Long-qi, Chen Ming-gang, Xiao Shu-hua, Liu Shu-xian, David Blair, Donald P. McManus, George M. Davis. [wwwnc.cdc.gov/eid/article/3/3/pdfs/97-0306.pdf Emerging and Reemerging Helminthiases and the Public Health of China]. Emerging Infectious Diseases. July–September 1997;3(3):303-310.
External links
- Center for Disease Control paragonimiasis web article
- The Human Lung Fluke - Paragonimus westermani at Cambridge Schistosomiasis Research Group