Meropenem
{{DrugProjectFormSinglePage |authorTag=Stefano Giannoni [1] |genericName=Meropenem |aOrAn=a |drugClass=Carbapenem |indicationType=treatment |indication=complicated skin and skin structure infections, intra-abdominal infections, bacterial meningitis (pediatric patients). |blackBoxWarningTitle=TITLE |blackBoxWarningBody=Condition Name: (Content) |fdaLIADAdult=====Skin and Skin Structure Infections====
- Dosage: 500 mg IV every 8 hours.
Is indicated as a single agent therapy for the treatment of complicated skin and skin structure infections due to:
- Staphylococcus aureus (methicillin-susceptible isolates only)
- Streptococcus pyogenes
- Streptococcus agalactiae
- Viridans group streptococci
- Enterococcus faecalis (vancomycin-susceptible isolates only)
- Pseudomonas aeruginosa
- Escherichia coli
- Proteus mirabilis
- Bacteroides fragilis
- Peptostreptococcus species
Intra-abdominal Infections
- 1 g IV every 8 hours
Is indicated as a single agent therapy for the treatment of complicated appendicitis and peritonitis caused by:
- Viridans group streptococci
- Escherichia coli
- Klebsiella pneumoniae
- Pseudomonas aeruginosa
- Bacteroides fragilis
- B. thetaiotaomicron
- Peptostreptococcus species
Meropenem for injection (I.V.) should be administered by intravenous infusion over approximately 15 to 30 minutes. Doses of 1 g may also be administered as an intravenous bolus injection (5 to 20 mL) over approximately 3-5 minutes.
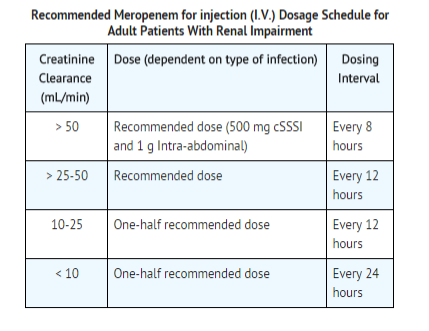
Use in Adult Patients with Renal Impairment
- Dosage should be reduced in patients with creatinine clearance of 50 mL/min or less.
- When only serum creatinine is available, the following formula (Cockcroft and Gault equation) may be used to estimate creatinine clearance.

There is inadequate information regarding the use of Meropenem for injection (I.V.) in patients on hemodialysis or peritoneal dialysis. |offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Meropenem in adult patients. |offLabelAdultNoGuideSupport=====Cystic Fibrosis====
- 120 mg/kg IV t.i.d. (maximum 2 g/dose)[1]
- In combination with tobramycin
Nosocomial Pneumonia
- 1 g IV t.i.d.[2]
- As monotherapy
Febrile Neutropenia
- 1 g t.i.d.[3]
- As monotherapy.
|fdaLIADPed=====Skin and Skin Structure Infections (Pediatric Patients ≥ 3 Months only)====
- 10m/kg IV every 8 hours.
- Up to a maximum Dose of 500 mg
- Pediatric patients weighing over 50 kg should be administered Meropenem for injection (I.V.) at a dose of 500 mg every 8 hours for complicated skin and skin structure infections.
Is indicated as a single agent therapy for the treatment of complicated skin and skin structure infections due to:
- Staphylococcus aureus (methicillin-susceptible isolates only)
- Streptococcus pyogenes
- Streptococcus agalactiae
- Viridans group streptococci
- Enterococcus faecalis (vancomycin-susceptible isolates only)
- Pseudomonas aeruginosa
- Escherichia coli
- Proteus mirabilis
- Bacteroides fragilis
- Peptostreptococcus species
Intra-abdominal Infections (Pediatric Patients ≥ 3 Months only)
- 20 mg/kg IV every 8 hours
- Up to a maximum dose of 1g
- Pediatric patients weighing over 50 kg should be administered Meropenem for injection (I.V.) at a dose of 1 g every 8 hours for intra-abdominal infections.
Is indicated as a single agent therapy for the treatment of complicated appendicitis and peritonitis caused by:
- Viridans group streptococci
- Escherichia coli
- Klebsiella pneumoniae
- Pseudomonas aeruginosa
- Bacteroides fragilis
- B. thetaiotaomicron
- Peptostreptococcus species
Bacterial Meningitis (Pediatric Patients ≥ 3 Months only)
- 40 mg IV every 8 hours.
- Up to a maximum of 2 g.
Pediatric patients weighing over 50 kg should be administered Meropenem for injection (I.V.) at a dose of 2 g every 8 hours for meningitis. Meropenem for injection (I.V.) is indicated as a single agent therapy for the treatment of bacterial meningitis caused by:
Meropenem for injection (I.V.) should be given as intravenous infusion over approximately 15 to 30 minutes or as an intravenous bolus injection (5 to 20 mL) over approximately 3-5 minutes.
There is limited safety data available to support the administration of a 40 mg/kg (up to a maximum of 2 g) bolus dose. There is no experience in pediatric patients with renal impairment. |offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Meropenem in pediatric patients. |offLabelPedNoGuideSupport=====Cystic Fibrosis====
- 120 mg/kg IV t.i.d. (maximum 2 g/dose)[1]
- In combination with tobramycin
Febrile Neutropenia
- 60 mg/kg/day t.i.d.[4]
- As monotherapy
|contraindications=*Hypersensitivity to any component of this product or to other drugs in the same class
- Demonstrated anaphylactic reactions to β-lactams.
|warnings=====Hypersensitivity Reactions====
- Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients receiving therapy with β-lactams.
- These reactions are more likely to occur in individuals with a history of sensitivity to multiple allergens.
- There have been reports of individuals with a history of penicillin hypersensitivity who have experienced severe hypersensitivity reactions when treated with another β-lactam.
- Before initiating therapy with Meropenem for injection (I.V.), careful inquiry should be made concerning previous hypersensitivity reactions to penicillins, cephalosporins, other β-lactams, and other allergens. *If an allergic reaction to Meropenem for injection (I.V.) occurs, discontinue the drug immediately.
- Serious anaphylactic reactions require immediate emergency treatment with epinephrine, oxygen, intravenous steroids, and airway management, including intubation. Other therapy may also be administered as indicated.
Seizure Potential
- Seizures and other adverse CNS experiences have been reported during treatment with Meropenem for injection (I.V.).
- These experiences have occurred most commonly in patients with CNS disorders (e.g., brain lesions or history of seizures) or with bacterial meningitis and/or compromised renal function.
- During clinical investigations, 2904 immunocompetent adult patients were treated for non-CNS infections with the overall seizure rate being 0.7% (based on 20 patients with this adverse event).
- All meropenem-treated patients with seizures had pre-existing contributing factors.
- Among these are included prior history of seizures or CNS abnormality and concomitant medications with seizure potential.
- Dosage adjustment is recommended in patients with advanced age and/or reduced renal function.
- Close adherence to the recommended dosage regimens is urged, especially in patients with known factors that predispose to convulsive activity.
- Anti-convulsant therapy should be continued in patients with known seizure disorders.
- If focal tremors, myoclonus, or seizures occur, patients should be evaluated neurologically, placed on anti-convulsant therapy if not already instituted, and the dosage of Meropenem for injection (I.V.) re-examined to determine whether it should be decreased or the antibiotic discontinued.
Interaction with Valproic Acid
- Case reports in the literature have shown that co-administration of carbapenems, including meropenem, to patients receiving valproic acid or divalproex sodium results in a reduction in valproic acid concentrations.
- The valproic acid concentrations may drop below the therapeutic range as a result of this interaction, therefore increasing the risk of breakthrough seizures.
- Increasing the dose of valproic acid or divalproex sodium may not be sufficient to overcome this interaction.
- The concomitant use of meropenem and valproic acid or divalproex sodium is generally not recommended.
- Antibacterials other than carbapenems should be considered to treat infections in patients whose seizures are well controlled on valproic acid or divalproex sodium.
- If administration of Meropenem for injection (I.V.) is necessary, supplemental anti-convulsant therapy should be considered.
Clostridium difficile–Associated Diarrhea
- Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Meropenem for injection (I.V.), and may range in severity from mild diarrhea to fatal colitis.
- Treatment with ntibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
- C. difficile produces toxins A and B which contribute to the development of CDAD.
- Hypertoxin producing isolates of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy.
- CDAD must be considered in all patients who present with diarrhea following antibiotic use.
- Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
- If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued.
- Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Development of Drug-Resistant Bacteria
- Prescribing Meropenem for injection (I.V.) in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Overgrowth of Nonsusceptible Organisms
- As with other broad-spectrum antibiotics, prolonged use of meropenem may result in overgrowth of nonsusceptible organisms.
- Repeated evaluation of the patient is essential.
- If superinfection does occur during therapy, appropriate measures should be taken.
Laboratory Tests
- While Meropenem for injection (I.V.) possesses the characteristic low toxicity of the beta-lactam group of antibiotics, periodic assessment of organ system functions, including renal, hepatic, and hematopoietic, is advisable during prolonged therapy.
Patients with Renal Impairment
- In patients with renal impairment, thrombocytopenia has been observed but no clinical bleeding reported.
Dialysis
- There is inadequate information regarding the use of Meropenem for injection (I.V.) in patients on hemodialysis or peritoneal dialysis.
Potential for Neuromotor Impairment
- Patients receiving Meropenem for injection (I.V.) on an outpatient basis may develop adverse events such as seizures, headaches and/or paresthesias that could interfere with mental alertness and/or cause motor impairment.
- Until it is reasonably well established that Meropenem for injection (I.V.) is well tolerated, patients should not operate machinery or motorized vehicles.
|clinicalTrials=Because clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in the [[clinical trials of another drug and may not reflect the rates observed in practice.
Adult Patients
- During clinical investigations, 2904 immunocompetent adult patients were treated for non-CNS infections with Meropenem for injection (I.V.) (500 mg or 1000 mg every 8 hours).
- Deaths in 5 patients were assessed as possibly related to meropenem; 36 (1.2%) patients had meropenem discontinued because of adverse events.
- Many patients in these trials were severely ill and had multiple background diseases, physiological impairments and were receiving multiple other drug therapies.
- In the seriously ill patient population, it was not possible to determine the relationship between observed adverse events and therapy with Meropenem for injection (I.V.).
The following adverse reaction frequencies were derived from the clinical trials in the 2904 patients treated with Meropenem for injection (I.V.)
Local Adverse Reactions
Local adverse reactions that were reported irrespective of the relationship to therapy with Meropenem for injection (I.V.) were as follows:
- Inflammation at the injection site 2.4%
- Injection site reaction 0.9%
- Phlebitis/thrombophlebitis 0.8%
Systemic Adverse Reactions
Systemic adverse reactions that were reported irrespective of the relationship to Meropenem for injection (I.V.) occurring in greater than 1% of the patients were diarrhea (4.8%), nausea/vomiting (3.6%), headache (2.3%), rash (1.9%), sepsis (1.6%), constipation (1.4%), apnea (1.3%), shock (1.2%), and pruritus (1.2%).
Additional systemic adverse reactions that were reported irrespective of relationship to therapy with Meropenem for injection (I.V.) and occurring in less than or equal to 1% but greater than 0.1% of the patients are listed below within each body system in order of decreasing frequency:
- Bleeding events were seen as follows: gastrointestinal hemorrhage (0.5%), melena (0.3%), epistaxis (0.2%), hemoperitoneum (0.2%), summing to 1.2%.
Body as a Whole
- Pain
- Abdominal pain
- Chest pain
- Fever
- Back pain
Abdominal enlargement Chills
- Pelvic pain
Cardiovascular
- Heart failure
- Heart arrest
- Tachycardia
- Hypertension
- Myocardial infarction
- Pulmonary embolus
- Bradycardia
- Hypotension
- Syncope
Digestive System
- Oral moniliasis
Anorexia Cholestatic jaundice/jaundice Flatulence Ileus Hepatic failure Dyspepsia Intestinal obstruction
Hemic/Lymphatic
- Anemia
- Hypochromic anemia
- Hypervolemia
Metabolic/Nutritional
- Peripheral edema
- Hypoxia
Nervous System
Insomnia Agitation/delirium Confusion Dizziness Seizure Nervousness Paresthesia Hallucinations Somnolence Anxiety Depression Asthenia
Respiratory: Respiratory disorder Dyspnea Pleural effusion Asthma Cough increased Lung edema
Skin and Appendages
- Urticaria sweating
- Skin ulcer
Urogenital System
- Dysuria
- Kidney failure
- Vaginal moniliasis
- Urinary incontinence
Adverse Laboratory Changes
Adverse laboratory changes that were reported irrespective of relationship to Meropenem for injection (I.V.) and occurring in greater than 0.2% of the patients were as follows:
Hepatic
- Increased SGPT (ALT), SGOT (AST), alkaline phosphatase, LDH, and bilirubin
Hematologic
- Increased platelets
- Increased eosinophils
- Decreased platelets
- Decreased hemoglobin
- Decreased hematocrit
- Decreased WBC
- Shortened prothrombin time and shortened partial thromboplastin time, *Leukocytosis
- Hypokalemia
Renal
- Increased creatinine
- Increased BUN
For patients with varying degrees of renal impairment, the incidence of heart failure, kidney failure, seizure and shock reported irrespective of relationship to Meropenem for injection (I.V.), increased in patients with moderately severe renal impairment (creatinine clearance > 10 to 26 mL/min).
Urinalysis
- Presence of red blood cells
- Pain at the injection site 0.4%
- Edema at the injection site 0.2%
|alcohol=Alcohol-Meropenem interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. |brandNames=*Merrem IV }}
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [3]
Overview
Meropenem is an ultra-broad spectrum injectable antibiotic used to treat a wide variety of infections, including meningitis and pneumonia. It is a beta-lactam and belongs to the subgroup of carbapenem, similar to imipenem and ertapenem. Meropenem gained FDA approval in July 1996. It penetrates well into many tissues and body fluids including the cerebrospinal fluid,bile, heart valves, lung, and peritoneal fluid.[5]
Category
Carbapenem
US Brand Names
MERREM®
FDA Package Insert
Description | Clinical Pharmacology | Microbiology | Indications and Usage | Contraindications | Warnings | Precautions | Adverse Reactions | Overdosage | Clinical Studies | Dosage and Administration | Compatibility, Reconstitution, and Stability | How Supplied | Labels and Packages
Mechanism of action
Meropenem is bactericidal except against Listeria monocytogenes where it is bacteriostatic. It inhibits bacterial wall synthesis like other beta-lactam antibiotics. In contrast to other beta-lactams, it is highly resistant to degradation by beta-lactamase or cephalosporinase. Resistance generally arises due to mutations in penicillin binding proteins, production of metallo-beta-lactamases, or resistance to diffusion across the bacterial outer membrane.[6] Unlike imipenem, it is stable to dehydropeptidase-1 and can therefore be given without cilastatin.
References
- ↑ 1.0 1.1 Latzin P, Fehling M, Bauernfeind A, Reinhardt D, Kappler M, Griese M (2008). "Efficacy and safety of intravenous meropenem and tobramycin versus ceftazidime and tobramycin in cystic fibrosis". J Cyst Fibros. 7 (2): 142–6. doi:10.1016/j.jcf.2007.07.001. PMID 17766190.
- ↑ Alvarez Lerma F, Serious Infection Study Group (2001). "Efficacy of meropenem as monotherapy in the treatment of ventilator-associated pneumonia". J Chemother. 13 (1): 70–81. doi:10.1179/joc.2001.13.1.70. PMID 11233804.
- ↑ "Equivalent efficacies of meropenem and ceftazidime as empirical monotherapy of febrile neutropenic patients. The Meropenem Study Group of Leuven, London and Nijmegen". J Antimicrob Chemother. 36 (1): 185–200. 1995. PMID 8537265.
- ↑ Oguz A, Karadeniz C, Citak EC, Cil V, Eldes N (2006). "Experience with cefepime versus meropenem as empiric monotherapy for neutropenia and fever in pediatric patients with solid tumors". Pediatr Hematol Oncol. 23 (3): 245–53. doi:10.1080/08880010500506867. PMID 16517540.
- ↑ AHFS DRUG INFORMATION® 2006 (2006 ed ed.). American Society of Health-System Pharmacists. 2006.
- ↑ Mosby's Drug Consult 2006 (16 ed ed.). Mosby, Inc. 2006.