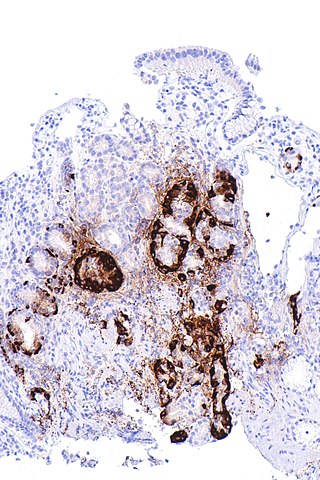
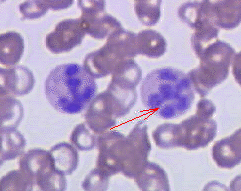
Chronic myelogenous leukemia overview
|
Chronic myelogenous leukemia Microchapters |
|
Differentiating Chronic myelogenous leukemia from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Chronic myelogenous leukemia overview On the Web |
|
American Roentgen Ray Society Images of Chronic myelogenous leukemia overview |
|
Directions to Hospitals Treating Chronic myelogenous leukemia |
|
Risk calculators and risk factors for Chronic myelogenous leukemia overview |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Mohamad Alkateb, MBBCh [2] "sandbox:SN"
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]; Associate Editor(s)-in-Chief:
Overview
Pernicious anemia (also called Addison's anemia) is a type of red blood cell disorder caused by impaired vitamin B12 metabolism. Vitamin B12 is primarily absorbed by the small intestine, after being bound to intrinsic factor secreted by parietal cells of gastric mucosa. When this process is disrupted by conditions like atrophic gastritis, celiac disease, small bowel resection etc, B12 deficiency ensues.
Historical perspective
- Pernicious anemia was first discovered by Thomas Addison, hence it is also known as addison's anemia.
- Loss of life from large volume blood loss in the people fighting in the first world war inspired George Whipple to investigate blood forming components such as arsenic, iron pills etc, but found liver to be the most effective. He bled dogs until they had clinical anemia and fed them cooked liver which showed an improvement in symptoms and hematopoeisis. [1]
- In 1948, Smith, Rickles et al., isolated the anti-pernicious factor from liver extract and named it Vitamin B12. They showed that even small amounts of this factor can be used to treat and to prevent pernicious anemia. [2]
Pathophysiology
Vitamin B12 is an essential vitamin for humans and animals because we cannot synthesise it on our own. B12 is a cofactor in DNA synthesis and other important biochemical reactions. Vitamin B12 deficiency manifests as anemia because hematopoetic stem cells in the bone marrow which are rapidly dividing need B12 for division and DNA production. This process is impaired leading to ineffective hematopoeisis. Vitamin B12 is also necessary for production of myelin which is an important component in the covering sheath of nerves. Deficiency results in improper nerve conduction due to nerve destabilisation. [3]
Physiology
- Vitamin B12 is also called cobalamin because it contains cobalt at the core of its structure. Dietary sources of vitamin B12 include meat, fish and eggs.[4]
- When consumed through its dietary source, B12 is bound to protein till it enters the stomach.
- In the stomach, B12 is uncoupled from its carrier protein due to the presence of gastric acid, which is why vitamin B12 deficiency is so commonly seen among those on chronic antacid medication. [5]
- Once in the stomach, it is then bound to gastric R binder, a glycoprotein secreted by the salivary glands till it reaches the duodenum.[6]
- In the duodenum and jejunum, the pancreatic enzymes digest the gastric R binder and cobalamin is bound to intrinsic factor (IF).
- Intrinsic factor is secreted by the gastric parietal cells. Once bound to IF, vitamin B12 travels up to the ileum where IF is removed and B12 binds with carrier proteins called transcobalamins and this complex is taken up by the liver and bone marrow, among other tissues.
- Inside the cells, the transcobalamin-B12 complex is dissolved and cobalamin is reduced to methylcobalamin which serves as a cofactor and coenzyme in many important biochemical reactions[7].
The two major reactions involving B12 in the human body are:
- Vitamin B12 in the from of cyanocobalamin is required in the synthesis of methionine. Methionine is produced from homocysteine and is catalysed by the enzyme methionine synthase. This enzyme utilises cyanocobalamin as a cofactor. Deficiency of vitamin B12 causes a decreased production of methionine and buildup of homocysteine. Hyperhomocysteinemia is implicated as a risk factor in cardiovascular disease.[8]
- The Kreb's cycle utilises vitamin B12 in the reaction converting methylmalonyl-CoA to succinyl-CoA. Thus vitamin B12 deficiency causes a buildup of methylmalonic acid, the substrate for the enzyme methylmalonyl coenzyme A mutase. Methylmalonic acid levels are elevated in the urine of people affected with pernicious anemia and other forms of B12 deficiency.
Storage
The human body can store anywhere from 2-5mg of vitamin B12. Most of this is stored in the liver and is recycled via enterohepatic circulation.
Pathogenesis
Pernicious anemia is a type of megaloblastic anemia caused due to improper vitamin B12 absorption by the body. Impaired absorption occurs because of deficiency of intrinsic factor which is produced by the parietal cells of the stomach. The etiology of pernicious anemia can be due to autoimmune causes or genetic disease. In autoimmune disease, the antibodies attack most of the gastric mucosa, but the antrum is spared.
Autoimmune causes of pernicious anemia
This is the most common cause of pernicious anemia. In autoimmune pernicious anemia, the body produces antibodies against parietal cells or intrinsic factor.
- Antibodies against parietal cells of the gastric mucosa work to inhibit the H+/K(+)-ATPase which is the proton pump present in the parietal cells. The proton pump serves as an auto antigen and activates the cytotoxic CD4+ T cells which proceed to destroy gastric mucosal cells.[9][10]
- Intrinsic factor antibodies are present in fewer cases of pernicious anaemia but are highly specific. There are 2 types of IF antibodies. They prevent the binding and absorption of cobalamin in the ileum via its receptor.[11]
Clinical features
- The symptoms of pernicious anemia take months, and often years to manifest. Patients most commonly present with symptoms of anemia like lightheadedness, dizziness, shortness of breath etc. The population affected with pernicious anemia is usually the elderly (>60 years) owing to its insidious onset.
- Pernicious anemia has hematological, gastrointestinal and neurological manifestations.
- Hematological signs are the earliest manifestation of the disease while neurological signs are seen much later.
- Patients with pernicious anemia usually have very low levels of hydrochloric acid in the stomach (achlorhydria) and high levels on gastrin (hypergastrinemia).
Differentiating pernicious anemia from other diseases
Pernicious anemia shares many similarities with other forms of megaloblastic anemia like B12 and folate deficiency.
- Vitamin B12 deficiency due to insufficient intake (eg veganism) has all the features of pernicious anemia like megaloblasts, hypersegmented neutrophils, neuropsychiatric manifestations. But atrophic gastritis is absent, so achlorhydria, parietal cell antibodies or IF antibodies are absent. Intrinsic factor levels are also normal.[6]
- Folic acid deficiency also results in megaloblastic anemia and similar hematological changes as pernicious anemia, but urinary excretion of methylmalonic acid is absent, so are features of pernicious anemia like achlorhydria, antibodies and normal IF levels.
- Ileal resection causes B12 deficiency due to decreased absorption.
- Certain drugs such as methotrexate, azathioprine cause folate deficiency and result in megaloblastic anemia. This is usually seen in patients taking chemotherapy or other chronic conditions such as rheumatoid arthritis. [12]
- Chronic proton pump inhibitor therapy also results in B12 deficiency as vitamin B12 cannot dissociate from its carrier protein in the absence of an acidic environment.[13]
- Long term use of metformin, such as in diabetics, is linked to vitamin B12 deficiency and symptoms similar to pernicious anemia, but this can be differentiated from pernicious anemia as it is seen in diabetics on chronic therapy.[14]
Associated Conditions
People affected with pernicious anemia might have other coexisting autoimmune conditions such as autoimmune thyroiditis, autoimmune diabetes, vitiligo etc. Autoimmune thyroiditis is most commonly seen in patients with pernicious anemia, particularly females. HLA DR3 has been implicated in the development of autoimmune diseases such as pernicious anemia[15].
Epidemiology and demographics
- Pernicious anemia is a disease of the elderly. The mean age of patients who are symptomatic is >60.[16]
- An exception is the genetic form of the disease which is a congenital deficiency of intrinsic factor and is seen in children <10 years of age.
- Men and women are equally affected
- Prevalence of pernicious anemia is estimated at 0.1% of the population.[17]
Genetics
- Some forms of pernicious anemia are congenital and a genetic link has been postulated because of a higher incidence in certain populations.
- Affected people have a complete or near total absence of intrinsic factor and the presence of antibodies against intrinsic factor.
- The genetic variant is transmitted through an autosomal recessive pattern.[18]
Risk factors
- People who have autoimmune conditions like diabetes mellitus, autoimmune thyroiditis are at higher risk of developing pernicious anemia.
Natural History, Complications and Prognosis
- In most cases, patients affected with pernicious anemia remain asymptomatic for many years.
- Early manifestations include fatigue, shortness of breath, pallor and weakness.
- Long standing untreated pernicious anemia results in irreversible neurological damage such as subacute combined degeneration of the spinal cord.
- Neurological changes are irreversible once they set in and do not resolve with cobalamin supplementation.
Diagnosis
A diagnosis of pernicious anemia is made by a history and physical examination, along with hematological and neurological examination.
Diagnostic criteria
- The only specific criteria to diagnose pernicious anemia is an intrinsic factor output of less than 200U/h after pentagastrin stimulation, where normal levels would be >2000U/h. [19]
Symptoms
Symptoms of pernicious anemia are summarised below
| Hematological symptoms | Gastrointestinal symptoms | Neurological symptoms |
|---|---|---|
| Fatigue | Loss of appetite | Parasthesias |
| Weakness | Weight loss
|
Depression |
| Shortness of breath | Nausea | Gait problems |
| Dizziness | Burning sensation on tongue | Weakness |
| Tachycardia | Diarrhea | Loss of balance |
| Lightheadedness | Vomiting | Confusion |
Physical examination findings
Most important physical examination findings are the neurological findings of long standing B12 deficiency which leads to subacute combined degeneration of the spinal cord.
- Hematological signs include pallor and icterus.[20]
- Neurological signs: Vitamin B12 deficiency causes nerve demyelination. B12 deficiency also causes a buildup of methylmalonic acid which is toxic to neuronal cells and causes apoptosis.[21].
The main neurological manifestation of pernicious anemia and vitamin B12 deficiency is subacute combined degeneration. The posterior and lateral columns of the spinal cord are affected. Lateral column demyelination manifests as hyperreflexia and spasticity, while posterior column defects are loss of proprioception and vibration sense. Ataxia and loss of tandem gait are also manifestations of posterior column demyelination. Recreational or accidental inhalation of nitrous oxide gas (laughing gas) can precipitate subacute combined degeneration in people with low levels of vitamin B12.[22]
- Gastrointestinal signs: Upto 25% of people affected with pernicious anemia develop glossitis. The tongue appears red, "beefy" and smooth due to atrophy and blunting of the lingual papillae.[23]
Subacute combined degeneration

Laboratory findings
- The first step in diagnosis is a blood vitamin B12 level. Blood levels less than 200 pg/ml are seen in pernicious anemia.
- Intrinsic factor antibodies and Parietal cell antibodies.
- Low intrinsic factor level.[24]
- Gastric mucosal sampling shows parietal cell atrophy with antral sparing.[25]
- Increased level of gastrin.
- Increased levels of homocysteine and methylmalonyl-CoA.
- Decreased folate levels are seen due to "folate trapping" in the form of methyltetrahydrofolate.
Shilling Test
The Shilling test is no longer done to detect an IF deficiency but has historical importance. After a vitamin B12 deficiency is noted, the patient is given radioactively tagged cobalamin to take orally. Soon after this step, the patient is injected with unlabelled cobalamin intramuscularly. Urine is checked for radioactive cobalamin for the next 24 hours. In pernicious anemia, there is an intrinsic factor deficiency, therefore the orally consumed radioactive cobalamin will not be absorbed and can be detected in the urine. In the next step, the patient is given radioactive cobalamin along with intrinsic factor and their urine is checked for traces of radioactive cobalamin. Absence of radioactive cobalamin in the urine points to the deficiency of intrinsic factor in the patients stomach which is the cause of vitamin B12 deficiency[26]. If the cobalamin absorption does not increase even with intrinsic factor supplementation, patient can be given a course of antibiotics as bacterial overgrowth may hinder absorption.
Peripheral smear findings
- The most obvious peripheral smear finding is megaloblasts and macrocytes.
Megaloblastic anemia results due to the lagging behind of nuclear development when compared to cytoplasmic development. This is known as nuclear-cytoplasmic asynchrony. Such defective cells are destroyed in the bone marrow (intramedullary hemolysis).
- Decreased number of RBCs (erythopenia)
- Macrocytosis- the RBCs in pernicious anemia are very large. Macrocytosis is defined as cells that have an MCV >100 femtolitres (normal :80-100fL)
- Hypersegmented neutrophils : Neutrophils containing ≥ 6 lobes. [27]
- Poikilocytosis and anisocytosis
- Low reticulocyte count (reticulopenia)
- Howell-Jolly bodies
-
Atrophic gastritis
-
Hypersegmented neutrophil
Treatment
- Standard treatment for pernicious anemia is replacement of cobalamin via intramuscular injection. [28]
- 1000 mcg IM everyday for one week, followed by weekly injections the next month and then monthly once injections.
- Response to treatment is measured by an increase in reticulocyte count within 5 days of starting therapy.
- Patient also experience a sense of wellbeing shortly after beginning therapy.
- If reticulocytosis is not observed within the first week of therapy, other factors such as hypothyroidism, folate deficiency should be considered.
- Intramuscular therapy can be replaced by high dose oral therapy.[17]
- Neurological disease always warrants parenteral treatment.
- Within the first 3-4 weeks of treatment, marrow changes revert and there is resolution in macrocytosis.
- Most patients require lifelong monthly therapy.
- Routine follow up should be done with a CBC every few months.
- A small percentage of patients develop gastric carcinoma, particularly in the elderly. Regular surveillance helps in early detection and treatment. [29]
Prevention
- There is no primary preventive measure for pernicious anemia.
- Once sucessfully diagnosed and treated, patients with pernicious anemia are followed up every year for development of stomach cancer[30], or symptoms of anemia.
References
Overview
Chronic myelogenous leukemia (CML) is a form of leukemia characterized by the increased and unregulated growth of predominantly myeloid cells in the bone marrow and the accumulation of these cells in the blood. CML is a clonal bone marrow stem cell disorder in which proliferation of mature granulocytes (neutrophils, eosinophils, and basophils) and their precursors is the main finding. It is a type of myeloproliferative disease associated with a characteristic chromosomal translocation called the Philadelphia chromosome. Historically, it has been treated with chemotherapy, interferon, bone marrow transplantation, and targeted therapies, which was introduced at the beginning of the 21st century and have radically changed the management of chronic myelogenous leukemia. In 1960, the association of Philadelphia chromosome with the pathogenesis of chronic myelogenous leukemia was first discovered. In 1973, (9;22) translocation was first discovered. Chronic myelogenous leukemia may be classified according to the hematologic characteristics and laboratory findings into five subtypes. Chronic myelogenous leukemia is caused by a mutation in BCR-ABL gene. The most potent risk factor in the development of chronic myelogenous leukemia is ionizing radiation; for example, increased rates of CML were seen in people exposed to the atomic bombings of Hiroshima and Nagasaki. According to the American Cancer Society, screening for chronic myelogenous leukemia is not recommended. Chronic myelogenous leukemia may be classified into five phases: chronic phase, accelerated phase, blast crisis, relapsed or recurrent CML and refractory disease. Chronic myelogenous leukemia must be differentiated from leukemoid reaction, chronic neutrophilic leukemia, and acute myeloid leukemia. Medical therapies for chronic myelogenous leukemia include chemotherapy, stem cell transplant , and/or biological therapy. [31][32][33][34][35][36][37][38]
Historical Perspective
In the 1840s, the first cases of chronic myelogenous leukemia (splenomegaly with high leukocyte count) was reported in France, Germany, and Scotland. In 1960, the association of Philadelphia chromosome with the pathogenesis of chronic myelogenous leukemia was first discovered. In 1973, (9;22) translocation was first discovered. Definition of the breakpoint cluster region (BCR) on chromosome 22 was first reported in 1984 and the demonstration of the BCR-ABL transcript in CML was first discovered in 1985. From 1980 onwards allogeneic stem cell transplantation (SCT) became the treatment of choice for eligible patients. In 1998, the era of tyrosine kinase inhibitors (TKI) began.[39][40]
Classification
[35]Chronic myelogenous leukemia (CML) may be classified according to the hematologic characteristics and laboratory findings into five subtypes: Chronic granulocytic leukaemia (CGL) (95% of all CML), Juvenile CML (extremely rare), Chronic neutrophilic leukaemia (CNL) (extremely rare), Chronic myelomonocytic leukaemia (CMML), Atypical CML (aCML).[35]
Pathophysiology
[41]Chronic myeloid leukemia (CML), a myeloproliferative neoplasm, characterized by the unrestrained expansion of pluripotent bone marrow stem cells. The hallmark of CML is the formation of the Philadelphia chromosome resulting from the reciprocal t(9;22)(q34;q11.2), resulting in a derivative 9q+ and a small 22q-. results in a BCR-ABL fusion gene and production of a BCR-ABL fusion protein. The gene product of the BCR-ABL gene constitutively activates numerous downstream targets including c-myc, Akt and Jun, all of which cause uncontrolled proliferation and survival of CML cells.[42][43]
Causes
[31]Chronic myelogenous leukemia is caused by:
- First, an abnormal chromosome develops: In people with chronic myelogenous leukemia, the Philadelphia chromosome, named for the city where it was discovered, is present in the blood cells of 90 percent of people.
- Second, the abnormal chromosome creates a new gene: The Philadelphia chromosome creates a new gene called BCR-ABL. it contains instructions that tell the abnormal blood cell to produce too much of a protein called tyrosine kinase that promotes cancer by allowing certain blood cells to grow out of control.
- Third, the new gene allows too many diseased blood cells: When the bone marrow functions normally, it produces immature cells (blood stem cells) in a controlled way. These cells then specialize into the various types of blood cells that circulate in the body. In chronic myelogenous leukemia, this process doesn't work correctly and the tyrosine kinase caused by the BCR-ABL gene causes too many white blood cells. These diseased white blood cells build up in huge numbers, crowding out healthy blood cells and damaging the bone marrow.
Differentiating Chronic myelogenous leukemia from other Diseases
Chronic myelogenous leukemia must be differentiated from leukemoid reaction, chronic neutrophilic leukemia, and acute myeloid leukemia.[33]
Epidemiology and Demographics
.[44] .[45]The incidence of Chronic Myeloid Leukemia (CML) was estimated to be 1–2 cases per 100,000 individuals worldwide and accounts for 15% of adult leukemias. The peak age for the CML is 50 to 55 and some series report a median age of up to 67 years. Incidence in CML increases by age, at least up to 75–80 years and in children, is a very rare disease with an incidence of 0.6–1.2 million children/year. Males are more commonly affected with CML than females. The male-to-female ratio varying between 1.2 and 1.7 in different studies. The gender difference in incidence is less prominent in younger people.[38][37][46][47]
Risk Factors
[36][48]The most potent risk factor in the development of chronic myelogenous leukemia is ionizing radiation; for example, increased rates of CML were seen in people exposed to the atomic bombings of Hiroshima and Nagasaki. Other risk factors include older age, being male, formaldehyde, obesity, and smoking.Family history is not a risk factor and The chromosome mutation that leads to chronic myelogenous leukemia is believed to be acquired.[46]
Screening
According to the American Cancer Society, screening for chronic myelogenous leukemia is not recommended.[32]
Natural History, Complications and Prognosis
[45][31]If left untreated, majority of patients with chronic myelogenous leukemia may progress from a chronic phase where differentiation is reasonably well-maintained to blast or acute phase (BP) where differentiation is lost. the progression to BP occurs at a median of 3–5 years from diagnosis in untreated patients. Some complications of chronic myelogenous leukemia include fatigue, excess bleeding, enlarged spleen, and infection. Prognosis is generally poor, and the 5-year survival rate of patients with chronic myelogenous leukemia is approximately 59.9%.targeted therapy with small molecule tyrosine kinase inhibitors (TKIs) dramatically alter the natural history of the disease, improving 10-year overall survival (OS) from 20 to 80–90%.[49][50][51][42]
Diagnosis
Staging
[48][52]Chronic myelogenous leukemia may be classified according to the clinical characteristics and laboratory findings into five phases: chronic phase, accelerated phase, blast crisis, relapsed or recurrent CML and refractory disease. The earliest phase is the chronic phase and generally has the best response to treatment. The accelerated phase is a transitional phase and blastic phase is a aggressive phase that becomes life-threatening. Relapsed CML means that the number of blast cells in the blood and bone marrow increase after remission and finally, refractory disease means the leukemia did not respond to treatment.[37] [38]
History and Symptoms
Up to 50% of patients with CML are asymptomatic and clinical features, when present, are generally nonspecific. Common symptoms of CML include fatigue, weight loss, fever, malaise, easy satiety, and left upper quadrant fullness or pain. Less common symptoms of CML include bleeding, thrombosis, gouty arthritis, symptoms of hyperviscosity including priapism, retinal hemorrhages, and upper gastrointestinal ulceration and bleeding. Dyspnea, drowsiness, loss of coordination, and confusion due to sludging in the pulmonary or cerebral vessels, are uncommon symptoms. Headaches, bone pain, arthralgias, pain from splenic infarction, and fever are more frequent with CML transformation.PMID:24729196/PMID:26434969.[48]
Physical Examination
Patients with chronic myelogenous leukemia are usually well-appearing. Physical examination of patients with chronic myelogenous leukemia is usually remarkable for skin bruising, fever, splenomegaly, and lymphadenopathy.[38][42]
Laboratory Findings
[48]Laboratory findings consistent with the diagnosis of chronic myelogenous leukemia in CBC include: thrombocytosis and/or marked leukocytosis (median of 100,000/µL) with a left shift, blasts usually number <2%, absolute basophilia is nearly universal, absolute eosinophilia, monocytosis and normal or elevated platelet count; thrombocytopenia suggests an alternative diagnosis or the presence of advanced stage. Elevated uric acid levels and elevated histamine levels due to basophilia are other laboratory findings.[42]
Chest X-Ray
Chest x-ray may be helpful in the diagnosis of chronic myelogenous leukemia. Findings on chest x-ray suggestive of chronic myelogenous leukemia include enlarged mediastinal lymph nodes, enlarged thymus gland, and pneumonia.[48]
Abdominal CT
Abdominal and chest CT scan may be helpful in the diagnosis of chronic myelogenous leukemia. Findings on CT scan suggestive of chronic myelogenous leukemia include enlarged lymph nodes.[48]
Brain MRI
Brain MRI may be helpful in the detection of brain metastasis in patients with chronic myelogenous leukemia.[48]
Abdominal Ultrasound
Abdominal ultrasound may be helpful in the diagnosis of chronic myelogenous leukemia. Findings on abdominal ultrasound suggestive of chronic myelogenous leukemia include enlarged lymph nodes and splenomegaly.[48]
Other Diagnostic Studies
[48]Other diagnostic studies for chronic myelogenous leukemia include bone marrow aspiration and biopsy, lumbar puncture, and lymph node biopsy. Genomic PCR, Southern blot assay, Reverse transcriptase (RT) PCR, Northern blot analysis, Western blot analysis or immunoprecipitation can be helpful in the diagnosis of chronic myelogenous leukemia; the gold standard diagnostic test in chronic myelogenous leukemia is cytogenetic analysis.[47][37]
Treatment
Medical Therapy
[31]Medical therapies for chronic myelogenous leukemia (CML) include chemotherapy, stem cell transplant , and/or biological therapy. With improved understanding of the nature of the BCR-ABL protein and its action as a tyrosine kinase, targeted therapies have been developed (the first of which was imatinib mesylate) which specifically inhibit the activity of the BCR-ABL protein. These tyrosine kinase inhibitors can induce complete remissions in chronic myelogenous leukemia, confirming the central importance of BCR-ABL as the cause of chronic myelogenous leukemia.[38][42][47]
Surgery
.[48]Surgery for Chronic Myeloid Leukemia - American Cancer Society
Surgery is not the first-line treatment option for patients with chronic myelogenous leukemia. Splenectomy is usually reserved for patients with enlarged spleen and it has no role in curing CML.
Primary Prevention
There are no primary preventive measures available for chronic myelogenous leukemia.[32]
Secondary Prevention
There are no secondary preventive measures available for chronic myelogenous leukemia.
References
- ↑ Sinclair L (2008). "Recognizing, treating and understanding pernicious anaemia". J R Soc Med. 101 (5): 262–4. doi:10.1258/jrsm.2008.081006. PMC 2376267. PMID 18463283.
- ↑ SMITH EL (1948). "Purification of anti-pernicious anaemia factors from liver". Nature. 161 (4095): 638. doi:10.1038/161638a0. PMID 18856623.
- ↑ Miles LM, Allen E, Clarke R, Mills K, Uauy R, Dangour AD (2017). "Impact of baseline vitamin B12 status on the effect of vitamin B12 supplementation on neurologic function in older people: secondary analysis of data from the OPEN randomised controlled trial". Eur J Clin Nutr. 71 (10): 1166–1172. doi:10.1038/ejcn.2017.7. PMID 28225050.
- ↑ Watanabe F (2007). "Vitamin B12 sources and bioavailability". Exp Biol Med (Maywood). 232 (10): 1266–74. doi:10.3181/0703-MR-67. PMID 17959839.
- ↑ Jung SB, Nagaraja V, Kapur A, Eslick GD (2015). "Association between vitamin B12 deficiency and long-term use of acid-lowering agents: a systematic review and meta-analysis". Intern Med J. 45 (4): 409–16. doi:10.1111/imj.12697. PMID 25583062.
- ↑ 6.0 6.1 Del Corral A, Carmel R (1990). "Transfer of cobalamin from the cobalamin-binding protein of egg yolk to R binder of human saliva and gastric juice". Gastroenterology. 98 (6): 1460–6. doi:10.1016/0016-5085(90)91076-i. PMID 2110915.
- ↑ Harrington DJ (2017). "Laboratory assessment of vitamin B12 status". J Clin Pathol. 70 (2): 168–173. doi:10.1136/jclinpath-2015-203502. PMID 27169753.
- ↑ Tinelli C, Di Pino A, Ficulle E, Marcelli S, Feligioni M (2019). "Hyperhomocysteinemia as a Risk Factor and Potential Nutraceutical Target for Certain Pathologies". Front Nutr. 6: 49. doi:10.3389/fnut.2019.00049. PMC 6491750. PMID 31069230.
- ↑ Callaghan JM, Khan MA, Alderuccio F, van Driel IR, Gleeson PA, Toh BH (1993). "Alpha and beta subunits of the gastric H+/K(+)-ATPase are concordantly targeted by parietal cell autoantibodies associated with autoimmune gastritis". Autoimmunity. 16 (4): 289–95. doi:10.3109/08916939309014648. PMID 7517707.
- ↑ Toh BH, Sentry JW, Alderuccio F (2000). "The causative H+/K+ ATPase antigen in the pathogenesis of autoimmune gastritis". Immunol Today. 21 (7): 348–54. doi:10.1016/s0167-5699(00)01653-4. PMID 10871877.
- ↑ Schade SG, Abels J, Schilling RF (1967). "Studies on antibody to intrinsic factor". J Clin Invest. 46 (4): 615–20. doi:10.1172/JCI105563. PMC 442045. PMID 6021209.
- ↑ Green R, Datta Mitra A (2017). "Megaloblastic Anemias: Nutritional and Other Causes". Med Clin North Am. 101 (2): 297–317. doi:10.1016/j.mcna.2016.09.013. PMID 28189172.
- ↑ Heidelbaugh JJ (2013). "Proton pump inhibitors and risk of vitamin and mineral deficiency: evidence and clinical implications". Ther Adv Drug Saf. 4 (3): 125–33. doi:10.1177/2042098613482484. PMC 4110863. PMID 25083257.
- ↑ Aroda VR, Edelstein SL, Goldberg RB, Knowler WC, Marcovina SM, Orchard TJ; et al. (2016). "Long-term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study". J Clin Endocrinol Metab. 101 (4): 1754–61. doi:10.1210/jc.2015-3754. PMC 4880159. PMID 26900641.
- ↑ Zulfiqar AA, Andres E (2017). "Association pernicious anemia and autoimmune polyendocrinopathy: a retrospective study". J Med Life. 10 (4): 250–253. PMC 5771255. PMID 29362601.
- ↑ Carmel R (1996). "Prevalence of undiagnosed pernicious anemia in the elderly". Arch Intern Med. 156 (10): 1097–100. PMID 8638997.
- ↑ 17.0 17.1 Andres E, Serraj K (2012). "Optimal management of pernicious anemia". J Blood Med. 3: 97–103. doi:10.2147/JBM.S25620. PMC 3441227. PMID 23028239.
- ↑ Gordon MM, Brada N, Remacha A, Badell I, del Río E, Baiget M; et al. (2004). "A genetic polymorphism in the coding region of the gastric intrinsic factor gene (GIF) is associated with congenital intrinsic factor deficiency". Hum Mutat. 23 (1): 85–91. doi:10.1002/humu.10297. PMID 14695536.
- ↑ Cattan D (2011). "Pernicious anemia: what are the actual diagnosis criteria?". World J Gastroenterol. 17 (4): 543–4. doi:10.3748/wjg.v17.i4.543. PMC 3027024. PMID 21274387.
- ↑ Seynabou F, Fatou Samba Diago N, Oulimata Diop D, Abibatou Fall S, Nafissatou D (2016). "Biermer anemia: Hematologic characteristics of 66 patients in a Clinical Hematology Unit at Senegal". Med Sante Trop. 26 (4): 402–407. doi:10.1684/mst.2016.0625. PMID 28073728.
- ↑ Han L, Wu S, Han F, Gu X (2015). "Insights into the molecular mechanisms of methylmalonic acidemia using microarray technology". Int J Clin Exp Med. 8 (6): 8866–79. PMC 4538064. PMID https://www.ncbi.nlm.nih.gov/pubmed/26309541 Check
|pmid=value (help). - ↑ Choi C, Kim T, Park KD, Lim OK, Lee JK (2019). "Subacute Combined Degeneration Caused by Nitrous Oxide Intoxication: A Report of Two Cases". Ann Rehabil Med. 43 (4): 530–534. doi:10.5535/arm.2019.43.4.530. PMC 6734019 Check
|pmc=value (help). PMID 31499607. - ↑ Stoopler ET, Kuperstein AS (2013). "Glossitis secondary to vitamin B12 deficiency anemia". CMAJ. 185 (12): E582. doi:10.1503/cmaj.120970. PMC 3761039. PMID 23359038.
- ↑ Lahner E, Annibale B (2009). "Pernicious anemia: new insights from a gastroenterological point of view". World J Gastroenterol. 15 (41): 5121–8. doi:10.3748/wjg.15.5121. PMC 2773890. PMID 19891010.
- ↑ Korman MG, Strickland RG, Hansky J (1972). "The functional 'G' cell mass in atrophic gastritis". Gut. 13 (5): 349–51. doi:10.1136/gut.13.5.349. PMC 1412218. PMID 5036089.
- ↑ "StatPearls". 2020. PMID 29939561.
- ↑ Farrelly SJ, O'Connor KA (2017). "Hypersegmented neutrophils and oval macrocytes in the setting of B12 deficiency and pancytopaenia". BMJ Case Rep. 2017. doi:10.1136/bcr-2016-218508. PMC 5612428. PMID 28821482.
- ↑ Annibale B, Lahner E, Fave GD (2011). "Diagnosis and management of pernicious anemia". Curr Gastroenterol Rep. 13 (6): 518–24. doi:10.1007/s11894-011-0225-5. PMID 21947876.
- ↑ Murphy G, Dawsey SM, Engels EA, Ricker W, Parsons R, Etemadi A; et al. (2015). "Cancer Risk After Pernicious Anemia in the US Elderly Population". Clin Gastroenterol Hepatol. 13 (13): 2282-9.e1-4. doi:10.1016/j.cgh.2015.05.040. PMC 4655146. PMID 26079040.
- ↑ Venerito M, Link A, Rokkas T, Malfertheiner P (2016). "Gastric cancer - clinical and epidemiological aspects". Helicobacter. 21 Suppl 1: 39–44. doi:10.1111/hel.12339. PMID 27531538.
- ↑ 31.0 31.1 31.2 31.3 National Cancer Institute. Physician Data Query Database 2015.http://www.cancer.gov/types/leukemia/hp/cml-treatment-pdq#section/_19
- ↑ 32.0 32.1 32.2 American Cancer Society.2015.http://www.cancer.org/cancer/leukemia-chronicmyeloidcml/detailedguide/leukemia-chronic-myeloid-myelogenous-detection
- ↑ 33.0 33.1 Gajendra S, Gupta R, Chandgothia M, Kumar L, Gupta R, Chavan SM (2014). "Chronic Neutrophilic Leukemia with V617F JAK2 Mutation". Indian J Hematol Blood Transfus. 30 (2): 139–42. doi:10.1007/s12288-012-0203-6. PMC 4022913. PMID 24839370.
- ↑ Goldman, John M. (2010). "Chronic Myeloid Leukemia: A Historical Perspective". Seminars in Hematology. 47 (4): 302–311. doi:10.1053/j.seminhematol.2010.07.001. ISSN 0037-1963.
- ↑ 35.0 35.1 35.2 Shepherd PC, Ganesan TS, Galton DA (1987). "Haematological classification of the chronic myeloid leukaemias". Baillieres Clin Haematol. 1 (4): 887–906. PMID 3332855.
- ↑ 36.0 36.1 Moloney WC (1987). "Radiogenic leukemia revisited". Blood. 70 (4): 905–8. PMID 3477299.
- ↑ 37.0 37.1 37.2 37.3 von Bubnoff N, Duyster J (February 2010). "Chronic myelogenous leukemia: treatment and monitoring". Dtsch Arztebl Int. 107 (7): 114–21. doi:10.3238/arztebl.2010.0114. PMC 2835925. PMID 20221270.
- ↑ 38.0 38.1 38.2 38.3 38.4 Jabbour E, Kantarjian H (May 2014). "Chronic myeloid leukemia: 2014 update on diagnosis, monitoring, and management". Am. J. Hematol. 89 (5): 547–56. doi:10.1002/ajh.23691. PMID 24729196.
- ↑ Nowell PC (August 2007). "Discovery of the Philadelphia chromosome: a personal perspective". J. Clin. Invest. 117 (8): 2033–5. doi:10.1172/JCI31771. PMC 1934591. PMID 17671636.
- ↑ Goldman JM (October 2010). "Chronic myeloid leukemia: a historical perspective". Semin. Hematol. 47 (4): 302–11. doi:10.1053/j.seminhematol.2010.07.001. PMID 20875546.
- ↑ Hehlmann R, Hochhaus A, Baccarani M; European LeukemiaNet (2007). "Chronic myeloid leukaemia". Lancet. 370 (9584): 342–50. PMID 17662883.
- ↑ 42.0 42.1 42.2 42.3 42.4 Thompson PA, Kantarjian HM, Cortes JE (October 2015). "Diagnosis and Treatment of Chronic Myeloid Leukemia in 2015". Mayo Clin. Proc. 90 (10): 1440–54. doi:10.1016/j.mayocp.2015.08.010. PMC 5656269. PMID 26434969.
- ↑ Jabbour E, Parikh SA, Kantarjian H, Cortes J (October 2011). "Chronic myeloid leukemia: mechanisms of resistance and treatment". Hematol. Oncol. Clin. North Am. 25 (5): 981–95, v. doi:10.1016/j.hoc.2011.09.004. PMC 4428141. PMID 22054730.
- ↑ Faderl S, Talpaz M, Estrov Z, Kantarjian HM (1999). "Chronic myelogenous leukemia: biology and therapy". Annals of Internal Medicine. 131 (3): 207–219. PMID 10428738.
- ↑ 45.0 45.1 Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2011, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission, posted to the SEER web site, April 2014.
- ↑ 46.0 46.1 Höglund M, Sandin F, Simonsson B (April 2015). "Epidemiology of chronic myeloid leukaemia: an update". Ann. Hematol. 94 Suppl 2: S241–7. doi:10.1007/s00277-015-2314-2. PMID 25814090.
- ↑ 47.0 47.1 47.2 Faderl S, Talpaz M, Estrov Z, Kantarjian HM (August 1999). "Chronic myelogenous leukemia: biology and therapy". Ann. Intern. Med. 131 (3): 207–19. PMID 10428738.
- ↑ 48.0 48.1 48.2 48.3 48.4 48.5 48.6 48.7 48.8 48.9 Canadian Cancer Society.2015.http://www.cancer.ca/en/cancer-information/cancer-type/leukemia-chronic-myelogenous-cml/staging/?region=ab
- ↑ Jabbour E, Kantarjian H (November 2012). "Chronic myeloid leukemia: 2012 update on diagnosis, monitoring, and management". Am. J. Hematol. 87 (11): 1037–45. doi:10.1002/ajh.23282. PMID 23090888.
- ↑ Radivoyevitch T, Jankovic GM, Tiu RV, Saunthararajah Y, Jackson RC, Hlatky LR, Gale RP, Sachs RK (March 2014). "Sex differences in the incidence of chronic myeloid leukemia". Radiat Environ Biophys. 53 (1): 55–63. doi:10.1007/s00411-013-0507-4. PMC 3943788. PMID 24337217.
- ↑ Deininger MW (2015). "Diagnosing and managing advanced chronic myeloid leukemia". Am Soc Clin Oncol Educ Book: e381–8. doi:10.14694/EdBook_AM.2015.35.e381. PMID 25993200.
- ↑ Vardiman J, Harris N, Brunning R (2002). "The World Health Organization (WHO) classification of the myeloid neoplasms". Blood. 100 (7): 2292–302. PMID 12239137. Retrieved 2007-09-22.