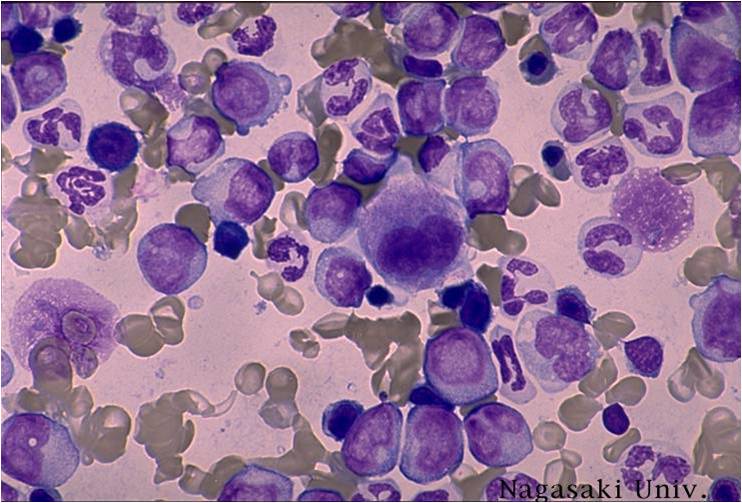
Chronic myelogenous leukemia
Template:DiseaseDisorder infobox
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [3] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Chronic myelogenous leukemia (CML) is a form of leukemia characterized by the increased and unregulated growth of predominantly myeloid cells in the bone marrow and the accumulation of these cells in the blood. CML is a clonal bone marrow stem cell disorder in which proliferation of mature granulocytes (neutrophils, eosinophils, and basophils) and their precursors is the main finding. It is a type of myeloproliferative disease associated with a characteristic chromosomal translocation called the Philadelphia chromosome. Historically, it has been treated with chemotherapy, interferon and bone marrow transplantation, although targeted therapies introduced at the beginning of the 21st century have radically changed the management of CML.
Epidemiology
CML occurs in all age groups, but most commonly in the middle-aged and elderly. Its annual incidence is 1–2 per 100,000 people, and slightly more men than women are affected. CML represents about 15–20% of all cases of adult leukemia in Western populations.[1] The only well-described risk factor for CML is exposure to ionizing radiation; for example, increased rates of CML were seen in people exposed to the atomic bombings of Hiroshima and Nagasaki.[2]
Signs and symptoms
Patients are often asymptomatic at diagnosis, presenting incidentally with an elevated white blood cell count on a routine laboratory test. In this setting, CML must be distinguished from a leukemoid reaction, which can have a similar appearance on a blood smear. Symptoms of CML may include: malaise, low-grade fever, gout, increased susceptibility to infections, anemia, and thrombocytopenia with easy bruising (although an increased platelet count (thrombocytosis) may also occur in CML). Splenomegaly may also be seen.[1][3]
Pathophysiology
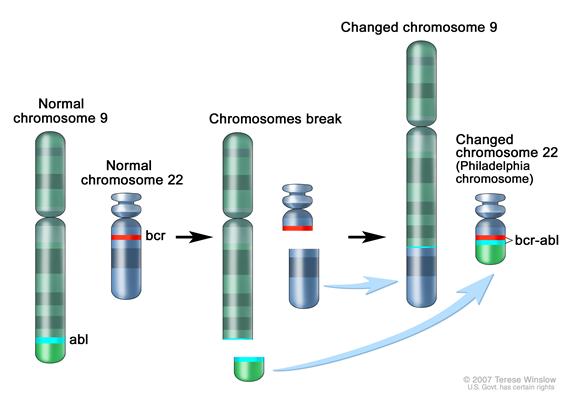
CML was the first malignancy to be linked to a clear genetic abnormality, the chromosomal translocation known as the Philadelphia chromosome. This chromosomal abnormality is so named because it was first discovered and described in 1960 by two scientists from Philadelphia, Pennsylvania: Peter Nowell of the University of Pennsylvania and David Hungerford of the Fox Chase Cancer Center. [4]
In this translocation, parts of two chromosomes (the 9th and 22nd by conventional karyotypic numbering) switch places. As a result, part of the BCR ("breakpoint cluster region") gene from chromosome 22 is fused with the ABL gene on chromosome 9. This abnormal "fusion" gene generates a protein of p210 or sometimes p185 weight (p is a weight measure of cellular proteins in kDa). Because abl carries a domain that can add phosphate groups to tyrosine residues (a tyrosine kinase), the bcr-abl fusion gene product is also a tyrosine kinase.[1][5]
The fused bcr-abl protein interacts with the interleukin 3beta(c) receptor subunit. The bcr-abl transcript is continuously active and does not require activation by other cellular messaging proteins. In turn, bcr-abl activates a cascade of proteins which control the cell cycle, speeding up cell division. Moreover, the bcr-abl protein inhibits DNA repair, causing genomic instability and making the cell more susceptible to developing further genetic abnormalities. The action of the bcr-abl protein is the pathophysiologic cause of chronic myelogenous leukemia. With improved understanding of the nature of the bcr-abl protein and its action as a tyrosine kinase, targeted therapies have been developed (the first of which was imatinib mesylate) which specifically inhibit the activity of the bcr-abl protein. These tyrosine kinase inhibitors can induce complete remissions in CML, confirming the central importance of bcr-abl as the cause of CML.[5]

Diagnosis
CML is often suspected on the basis on the complete blood count, which shows increased granulocytes of all types, typically including immature myeloid cells. Basophils and eosinophils are almost universally increased; this feature may help differentiate CML from a leukemoid reaction. A bone marrow biopsy is often performed as part of the evaluation for CML, but bone marrow morphology alone is insufficient to diagnose CML.[5][3]
Ultimately, CML is diagnosed by detecting the Philadelphia chromosome. This characteristic chromosomal abnormality can be detected by routine cytogenetics, by fluorescent in situ hybridization, or by PCR for the bcr-abl fusion gene.[3]
Controversy exists over so-called Ph-negative CML, or cases of suspected CML in which the Philadelphia chromosome cannot be detected. Many such patients in fact have complex chromosomal abnormalities which mask the (9;22) translocation, or have evidence of the translocation by FISH or RT-PCR in spite of normal routine karyotyping.[6] The small subset of patients without detectable molecular evidence of bcr-abl fusion may be better classified as having an undifferentiated myelodysplastic/myeloproliferative disorder, as their clinical course tends to be different from patients with CML.[7]
Phases of CML
CML is often divided into three phases based on clinical characteristics and laboratory findings. In the absence of intervention, CML typically begins in the chronic phase, and over the course of several years progresses to an accelerated phase and ultimately to a blast crisis. Blast crisis is the terminal phase of CML and clinically behaves like an acute leukemia. One of the drivers of the progression from chronic phase through acceleration and blast crisis is the acquisition of new chromosomal abnormalities (in addition to the Philadelphia chromosome).[1] Some patients may already be in the accelerated phase or blast crisis by the time they are diagnosed.[3]
Chronic phase
Approximately 85% of patients with CML are in the chronic phase at the time of diagnosis. During this phase, patients are usually asymptomatic or have only mild symptoms of fatigue or abdominal fullness. The duration of chronic phase is variable and depends on how early the disease was diagnosed as well as the therapies used. Ultimately, in the absence of curative treatment, the disease progresses to an accelerated phase.[3]
Accelerated phase
Criteria for diagnosing transition into the accelerated phase are somewhat variable; the most widely used criteria are those put forward by investigators at M.D. Anderson Cancer Center,[8] by Sokal et al,[9] and the World Health Organization.[10][7] The WHO criteria are perhaps most widely used, and include:
- 10–19% myeloblasts in the blood or bone marrow
- >20% basophils in the blood or bone marrow
- Platelet count <100,000, unrelated to therapy
- Platelet count >1,000,000, unresponsive to therapy
- Cytogenetic evolution with new abnormalities in addition to the Philadelphia chromosome
- Increasing splenomegaly or white blood cell count, unresponsive to therapy
The patient is considered to be in the accelerated phase if any of the above are present. The accelerated phase is significant because it signals that the disease is progressing and transformation to blast crisis is imminent.[7]
Blast crisis
Blast crisis is the final phase in the evolution of CML, and behaves like an acute leukemia, with rapid progression and short survival.[3] Blast crisis is diagnosed if any of the following are present in a patient with CML:[11]
- >20% myeloblasts or lymphoblasts in the blood or bone marrow
- Large clusters of blasts in the bone marrow on biopsy
- Development of a chloroma (solid focus of leukemia outside the bone marrow)
Treatment
Chronic phase
Chronic phase CML is treated with inhibitors of tyrosine kinase , the first of which was imatinib mesylate (marketed as Gleevec® or Glivec®; previously known as STI-571). In the past, antimetabolites (e.g. cytarabine, hydroxyurea), alkylating agents, interferon alfa 2b, and steroids were used, but these drugs have been replaced by imatinib. Imatinib was approved by the United States FDA in 2001 and specifically targets BCR/abl, the constitutively activated tyrosine kinase fusion protein caused by the Philadelphia chromosome translocation. It is better tolerated and more effective than previous therapies. Bone marrow transplantation was also used as initial treatment for CML in younger patients before the advent of imatinib, and while it can often be curative, there is a high rate of transplant-related mortality.[5]
To overcome imatinib resistance and to increase responsiveness to TK inhibitors, two novel agents are currently undergoing clinical trials. The first, dasatinib, is a TK inhibitor that blocks several oncogenic proteins and has been recently approved by the US FDA to treat CML patients who are either resistant to or intolerant of imatinib. Dasatanib and Imatinib resistance is caused by the T315I mutation. One drug to overcome this resistance is being developed by Merck (MK-0457, formerly known as VX-680), however, enrollments in this clinical trial are currently suspended, pending a full analysis of all efficacy and safety data [12]. Another drug in development for the T315I mutation is Omacetaxine (formerly known as Ceflatonin®). Clinical data from the first 21 patients enrolled in a Phase 2/3 trial were presented at the American Society of Hematology (ASH) Annual Meeting [13]. Another agent, nilotinib, is a selective kinase inhibitor, but is currently undergoing clinical development and testing. Nilotinib is designed to bind more tightly than imatinib to the Bcr-Abl abnormal fusion protein responsible for chronic myeloid leukemia. Stem cell transplantation is a secondary option for treatment of CML.[14][15]
In 2005 favourable results of vaccination were reported with the BCR/abl p210 fusion protein in patients with stable disease, with GM-CSF as an adjuvant.[16]
Blast crisis
Blast crisis carries all the symptoms and characteristics of either acute myelogenous leukemia or acute lymphoblastic leukemia, and has a very high mortality rate. This stage can most effectively be treated by a bone marrow transplant after high-dose chemotherapy. In young patients in the accelerated phase, a transplant may also be an option. However the likelihood of relapse after a bone marrow transplant is higher in patients in blast crisis or in the accelerated phase as compared to patients in the chronic phase.[14]
There are different types of treatment for patients with chronic myelogenous leukemia
Different types of treatment are available for patients with chronic myelogenous leukemia (CML). Some treatments are standard (the currently used treatment), and some are being tested in clinical trials. A treatment clinical trial is a research study meant to help improve current treatments or obtain information on new treatments for patients with cancer. When clinical trials show that a new treatment is better than the standard treatment, the new treatment may become the standard treatment. Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
Six types of standard treatment are used:
Tyrosine kinase inhibitor therapy
A drug called imatinib mesylate is used as initial treatment for certain types of chronic myelogenous leukemia in newly diagnosed patients. It blocks an enzyme called tyrosine kinase that causes stem cells to develop into more white blood cells (granulocytes or blasts) than the body needs. Another tyrosine kinase inhibitor called dasatinib is used to treat patients with certain types of CML that have progressed, and is being studied as an initial treatment.
Chemotherapy
Chemotherapy is a cancer treatment that uses drugs to stop the growth of cancer cells, either by killing the cells or by stopping them from dividing. When chemotherapy is taken by mouth or injected into a vein or muscle, the drugs enter the bloodstream and can reach cancer cells throughout the body (systemic chemotherapy). When chemotherapy is placed directly into the spinal column, an organ, or a body cavity such as the abdomen, the drugs mainly affect cancer cells in those areas (regional chemotherapy). The way the chemotherapy is given depends on the type and stage of the cancer being treated.
Biologic therapy
Biologic therapy is a treatment that uses the patient’s immune system to fight cancer. Substances made by the body or made in a laboratory are used to boost, direct, or restore the body’s natural defenses against cancer. This type of cancer treatment is also called biotherapy or immunotherapy.
High-dose chemotherapy with stem cell transplant
High-dose chemotherapy with stem cell transplant is a method of giving high doses of chemotherapy and replacing blood-forming cells destroyed by the cancer treatment. Stem cells (immature blood cells) are removed from the blood or bone marrow of the patient or a donor and are frozen and stored. After the chemotherapy is completed, the stored stem cells are thawed and given back to the patient through an infusion. These reinfused stem cells grow into (and restore) the body’s blood cells.
Donor lymphocyte infusion (DLI)
Donor lymphocyte infusion (DLI) is a cancer treatment that may be used after stem cell transplant. Lymphocytes (a type of white blood cell) from the stem cell transplant donor are removed from the donor’s blood and may be frozen for storage. The donor’s lymphocytes are thawed if they were frozen and then given to the patient through one or more infusions. The lymphocytes see the patient’s cancer cells as not belonging to the body and attack them.
Surgery
Splenectomy is surgery to remove the spleen.
Current Treatment Protocols for CML
Chronic Phase Chronic Myelogenous Leukemia
Treatment of chronic phase chronic myelogenous leukemia may include the following:
- Drug therapy with a tyrosine kinase inhibitor.
- High-dose chemotherapy with donor stem cell transplant.
- Biologic therapy (interferon) with or without chemotherapy.
- Chemotherapy.
- Splenectomy.
Accelerated Phase Chronic Myelogenous Leukemia
Treatment of accelerated phase chronic myelogenous leukemia may include the following:
- Stem cell transplant.
- Drug therapy with a tyrosine kinase inhibitor.
- Biologic therapy (interferon) with or without chemotherapy.
- High-dose chemotherapy.
- Chemotherapy.
- Transfusion therapy to replace red blood cells, platelets, and sometimes white blood cells, to relieve symptoms and improve quality of life.
Blastic Phase Chronic Myelogenous Leukemia
Treatment of blastic phase chronic myelogenous leukemia may include the following:
- Drug therapy with a tyrosine kinase inhibitor.
- Chemotherapy using one or more drugs.
- High-dose chemotherapy.
- Donor stem cell transplant.
- Chemotherapy as palliative therapy to relieve symptoms and improve quality of life.
Relapsed Chronic Myelogenous Leukemia
Treatment of relapsed chronic myelogenous leukemia may include the following:
- Drug therapy with a tyrosine kinase inhibitor.
- Donor stem cell transplant.
- Donor lymphocyte infusion.
- Biologic therapy (interferon).
Prognosis
In one analysis of several clinical studies, three different risk groups were identified based on a prognostic scoring system that includes several variables: age, spleen size, blast count, platelet count, eosinophil count and basophil count. In the lowest risk group, the median survival time was 98 months. In the middle group, the median was 65 months, and in the highest risk group, the median was about 42 months. Of all patients analyzed, the longest survival time was 117 months.[17] However, this study pre-dates the advent of treatments using targetted therapy. A follow-up on patients using imatinib published in the New England Journal of Medicine shows an overall survival rate of 89% after five years.[18]
The prognosis (chance of recovery) and treatment options depend on the following:
- The patient’s age.
- The phase of CML.
- The amount of blasts in the blood or bone marrow.
- The size of the spleen at diagnosis.
- The patient’s general health.
References
- ↑ 1.0 1.1 1.2 1.3 Faderl S, Talpaz M, Estrov Z, Kantarjian HM (1999). "Chronic myelogenous leukemia: biology and therapy". Annals of Internal Medicine. 131 (3): 207–219. PMID 10428738.
- ↑ Moloney WC (1987). "Radiogenic leukemia revisited". Blood. 70 (4): 905–908. PMID 3477299.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Tefferi A (2006). "Classification, diagnosis and management of myeloproliferative disorders in the JAK2V617F era". Hematology Am Soc Hematol Educ Program: 240–245. PMID 17124067.
- ↑ Nowell PC (2007). "Discovery of the Philadelphia chromosome: a personal perspective". Journal of Clinical Investigation. 117 (8): 2033–2035. PMID 17671636.
- ↑ 5.0 5.1 5.2 5.3 Hehlmann R, Hochhaus A, Baccarani M; European LeukemiaNet (2007). "Chronic myeloid leukaemia". Lancet. 370 (9584): 342–50. PMID 17662883.
- ↑ Savage DG; Szydlo RM; Goldman JM (1997). "Clinical features at diagnosis in 430 patients with chronic myeloid leukaemia seen at a referral centre over a 16-year period". Br J Haematol. 96 (1): 111–116. PMID 9012696.
- ↑ 7.0 7.1 7.2 Tefferi A, Thiele J, Orazi A, Kvasnicka HM, Barbui T, Hanson CA, Barosi G, Verstovsek S, Birgegard G, Mesa R, Reilly JT, Gisslinger H, Vannucchi AM, Cervantes F, Finazzi G, Hoffman R, Gilliland DG, Bloomfield CD, Vardiman JW (2007). "Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert pane". Blood. 110 (4): 1092–1097. PMID 17488875.
- ↑ Kantarjian H, Dixon D, Keating M, Talpaz M, Walters R, McCredie K, Freireich E (1988). "Characteristics of accelerated disease in chronic myelogenous leukemia". Cancer. 61 (7): 1441–6. PMID 3162181.
- ↑ Sokal J, Baccarani M, Russo D, Tura S (1988). "Staging and prognosis in chronic myelogenous leukemia". Semin Hematol. 25 (1): 49–61. PMID 3279515.
- ↑ Vardiman J, Harris N, Brunning R (2002). "The World Health Organization (WHO) classification of the myeloid neoplasms". Blood. 100 (7): 2292–302. PMID 12239137. Retrieved 2007-09-22.
- ↑ Karbasian Esfahani M, Morris EL, Dutcher JP, Wiernik PH (2006). "Blastic phase of chronic myelogenous leukemia". Current Treatment Options in Oncology. 7 (3): 189–199. PMID 16615875.
- ↑ FDANEWS.Nov 26 volume5 (230)
- ↑ Khoury, HJ. et al. Safety and Efficacy Study of Subcutenous Homoharringtonine(SC HHT) in Imatinib (IM)-Resistanct Chronic Myeloid Leukemia (CML) with the T315I Mutation-Intial report of a Phase II Trial (2007)Blood. 110(11):318a
- ↑ 14.0 14.1 Jabbour E, Cortes JE, Giles FJ, O'Brien S, Kantarjian HM (2007). "Current and emerging treatment options in chronic myeloid leukemia". Cancer. 109 (11): 2171–2181. PMID 17431887.
- ↑ Kimura S, Ashihara E, Maekawa T (2006). "New tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia". Current Pharmaceutical Biotechnology. 7 (5): 371–379. PMID 17076652.
- ↑ Bocchia M, Gentili S, Abruzzese E, Fanelli A, Iuliano F, Tabilio A, Amabile M, Forconi F, Gozzetti A, Raspadori D, Amadori S, Lauria F (2005). "Effect of a p210 multipeptide vaccine associated with imatinib or interferon in patients with chronic myeloid leukaemia and persistent residual disease: a multicentre observational trial". Lancet. 365 (9460): 657–62. PMID 15721470.
- ↑ Hasford J, Pfirrmann M, Hehlmann R, Allan NC, Baccarani M, Kluin-Nelemans JC, Alimena G, Steegmann JL, Ansari H (1998). "A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group". Journal of the National Cancer Institute. 90 (11): 850–858. PMID 9625174.
- ↑ Druker BJ, Guilhot F, O'Brien SG; et al. (2006). "Five-Year Follow-up of Patients Receiving Imatinib for Chronic Myeloid Leukemia". 355 (20): 2408–2417. doi:10.1056/NEJMoa062867. PMID 17151364.
External links
- The Leukemia & Lymphoma Society
- Blood & Marrow Transplant Information Network
- Association of Cancer Online Resource (ACOR) Leukemia Links
- Merck Manual:Chronic Myelocytic Leukemia (CML)
ar:ابيضاض الدم النقوي المزمن de:Chronische myeloische Leukämie ko:만성골수성백혈병 ms:Leukemia mielogenus kronik nl:Chronische myeloïde leukemie fi:Krooninen myelooinen leukemia sv:Kronisk myeloisk leukemi th:Chronic myelogenous leukemia