Fluorescence in situ hybridization
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
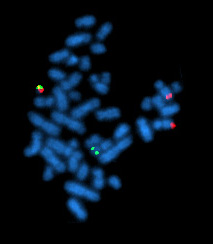
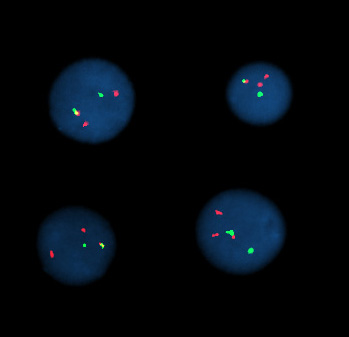
FISH (Fluorescent in situ hybridization) is a cytogenetic technique which can be used to detect and localize the presence or absence of specific DNA sequences on chromosomes. It uses fluorescent probes which bind only to those parts of the chromosome with which they show a high degree of sequence similarity. Fluorescence microscopy can be used to find out where the fluorescent probe bound to the chromosome. FISH is often used for finding specific features in DNA. These features can be used in genetic counseling, medicine, and species identification.
Probes
Probes are often derived from fragments of DNA that were isolated, purified, and amplified for use in the Human Genome Project. The size of the human genome is so large compared to the length that could be sequenced directly that it was necessary to divide the genome into fragments. The fragments were added into a framework that made it possible to use bacteria to replicate the fragments. The fragments were put into order by analyzing size-exclusion separation of enzymatically-digested fragments. Clonal populations of bacteria, each population maintaining a single artificial chromosome, are stored in various laboratories around the world. The artificial chromosomes (BAC) can be grown, extracted, and labeled, in any lab. These fragments are on the order of 100 thousand base-pairs, and are the basis for most FISH probes.
Preparation and Hybridization Process
First, a probe is constructed. The probe has to be long enough to hybridize specifically to its target (and not to similar sequences in the genome), but not too large to impede the hybridization process, and it should be tagged directly with fluorophores, with targets for antibodies or with biotin. This can be done in various ways, for example nick translation and PCR using tagged nucleotides.
Then, an interphase or metaphase chromosome preparation is produced. The chromosomes are firmly attached to a substrate, usually glass. Repetitive DNA sequences must be blocked by adding short fragments of DNA to the sample. The probe is then applied to the chromosome DNA and incubated for ~12 hours while hybridizing. Several wash steps remove all unhybridized or partially hybridized probes. The results are then visualized and quantified using a microscope that is capable of exciting the dye and recording images.
If the fluorescent signal is weak, amplification of the signal may be necessary in order to exceed the detection threshold of the microscope. The signal strength depends on many factors; probe labeling efficiency, the type of probe and the type of dye affect the fluorescent signal. Fluorescently-tagged antibodies or streptavidin are bound to the dye molecule. These secondary components are selected so that they have a strong signal.
Fibre FISH
An alternative to interphase or metaphase preparations, fibre FISH, interphase chromosomes are attached to a slide in such a way that they are stretched out in a straight line, rather than being tightly coiled, as in conventional FISH, or adopting a random conformation, as in interphase FISH. This is accomplished by applying mechanical shear along the length of the slide; either to cells which have been fixed to the slide and then lysed, or to a solution of purified DNA. A technique known as chromosome combing is increasingly used for this purpose. The extended conformation of the chromosomes allows dramatically higher resolution - even down to a few kilobases. The preparation of fibre FISH samples, although conceptually simple, is a rather skilled art, and only specialized laboratories use the technique routinely.
Variations on Probes and Analysis
FISH is a very general technique. It is often arbitrarily divided into more specific categories based on application, however each category is similar in that, in a chemical sense, the technique is the same; hybridization is the common denominator. The differences between the various FISH techniques are usually due to the construction and content of the fluorescently-labeled DNA probe. The size, overlap, colour, and mixture of the probes make possible all FISH techniques.
Probes size is important because longer probes hybridize more specifically than shorter probes. The overlap defines the resolution of detectable features. If the goal of an experiment is to detect the break point of a translocation, then the overlap of the probes—the degree to which one DNA sequence is contained in the adjacent probes—defines the minimum window in which the breakpoint occurs.
The mixture of probes determines the type of feature the probe can detect. Probes that hybridize along an entire chromosome are used to count the number of a certain chromosome, show translocations, or identify extra-chromosomal fragments of chromatin. This is often called "whole-chromosome painting." If every possible probe is used, every chromosome, (in essence the whole genome) would be marked fluorescently, which would not be particularly useful for determining features of individual sequences. A mixture of smaller probes can be created that are specific to a particular region (locus) of DNA; these mixtures are used to detect deletion mutations. When combined with a specific colour, a locus-specific probe mixture is used to detect very specific translocations. Special locus-specific probe mixtures are often used to count chromosomes, by binding to the centromeric regions of chromosomes, which are unique enough to identify each chromosome (with the exception of Chromosome 13, 14 21, 22.)
Because modern microscopes can detect a range of colours in fluorescent dyes, by using whole-chromosome probe mixtures and a variety of colours, each human chromosome can be identified (M-FISH). There are currently twice as many chromosomes than fluorescent dye colours. However, ratios of probe mixtures can be used to create additional colours. Much like comparative genomic hybridization, the probe mixture for the secondary colours is created by mixing the correct ratio of two sets of differently-labeled probes for the same chromosome. Differently-coloured probes can be used for the detection of translocations. Several techniques exploit the resolution limitations of microscopes to resolve spatial distributions of dye below a few hundred nanometers. Colours that are adjacent appear to overlap, and a secondary colour is observed.
In reciprocal translocations, where both breakpoints are known, locus-specific probes are made for it and part of the region one either side of breakpoint. In normal cells, two colours will be visible; in diseased cells such as is found in BCR/ABL translocations, the two dye colours overlap, and a third colour is observed. This technique is known as double-fusion FISH or D-FISH. In translocations where only one of the breakpoints is known or constant, locus-specific probes are made for one side of the break point and the other intact chromosome. In normal cells, the secondary colour is observed but only the primary colour is observed when the translocation occurs. This technique is known as "break-apart FISH".
Medical Applications
Often parents of children with a developmental delays want to know more about their child's conditions before choosing to have another child. These concerns can be addressed by analysis of the parents' and child's DNA. In cases where the child's developmental delay is not understood, the cause of it can be determined using FISH and cytogenetic techniques. Examples of diseases that are diagnosed using FISH include Prader-Willi syndrome, Angelman syndrome, chronic myelogenous leukemia, acute lymphoblastic leukemia, Cri-du-chat and Down syndrome.
In medicine, FISH can be used to form a diagnosis, evaluate prognosis, or to evaluate remission of a disease, such as cancer. Treatment can then be specifically tailored. A traditional exam and metaphase chromosome analysis is often unable to identify features that distinguish one disease from another, due to subtle chromosomal features; FISH can elucidate these differences. FISH can also be used to detect diseased cells more easily than standard Cytogenetic methods, which require dividing cells and requires labor and time intensive manual preparation and analysis of the slides by a technologist. FISH, on the other hand, does not require living cells and can be quantified automatically, a computer counts the fluorescent dots present. However, a trained technologist is required to distinguish subtle differences in banding patterns on bent and twisted metaphase chromosomes.
Species Identification
FISH is often used in clinical studies. If a patient is infected with a suspected pathogen, bacteria, from the patient's tissues or fluids, are typically grown on agar to determine the identity of the pathogen. Many bacteria, however, even well known species, do not grow well under laboratory conditions. FISH can be used to detect directly the presence of the suspect on small samples of patient's tissue.
FISH can also be used compare the genomes of two biological species, to deduce evolutionary relationships. A similar hybridization technique is called a zoo blot. Bacterial FISH probes are often primers for the 16s rRNA region.
FISH is widely used in the field of microbial ecology, to identify microorganisms. Biofilms, for example, are composed of complex (often) multi-species bacterial organizations. Preparing DNA probes for one species and performing FISH with this probe allows one to visualize the distribution of this specific species within the biofilm. Preparing probes (in two different colors) for two species allows to visualize/study co-localization of these two species in the biofilm, and can be useful in determining the fine architecture of the biofilm.
References
See Also
External Links
- Fluorescent+in+Situ+Hybridization at the US National Library of Medicine Medical Subject Headings (MeSH)
- Information on fiber FISH from the Olympus Corporation
- A guide to fiber FISH from Octavian Henegariu
- Fibre FISH protocol from the Human Genome Project at the Sanger Centre
- CARD-FISH, BioMineWiki