Aortic regurgitation pathophysiology
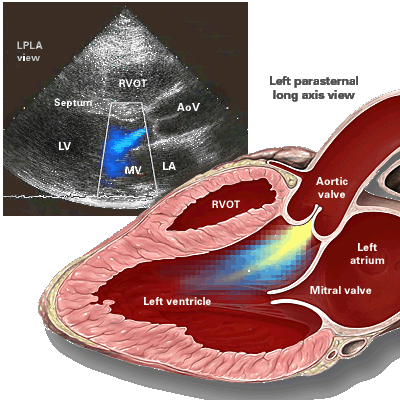
|
Aortic Regurgitation Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Acute Aortic regurgitation |
|
Chronic Aortic regurgitation |
|
Special Scenarios |
|
Case Studies |
|
Aortic regurgitation pathophysiology On the Web |
|
American Roentgen Ray Society Images of Aortic regurgitation pathophysiology |
|
Risk calculators and risk factors for Aortic regurgitation pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor-in-Chief: Varun Kumar, M.B.B.S., Lakshmi Gopalakrishnan, M.B.B.S., Mohammed A. Sbeih, M.D. [2]
Overview
In individuals with a normally functioning aortic valve, the valve is only open when the pressure in the left ventricle is higher than the pressure in the aorta. This allows the blood to be ejected from the left ventricle into the aorta during ventricular systole. The amount of blood that is ejected by the heart is known as the stroke volume. Under normal conditions, 50–70% of the blood in a filled left ventricle is ejected into the aorta to be used by the body (called the 'ejection fraction'). In aortic insufficiency (AI), when the pressure in the left ventricle falls below the pressure in the aorta, the aortic valve is not able to completely close. This causes a leaking of blood from the aorta into the left ventricle.
Pathophysiology
In aortic insufficiency (AI), some of the blood that was already ejected from the heart is regurgitating back into the heart. The percentage of blood that regurgitates back through the aortic valve due to AI is known as the regurgitant fraction. This regurgitant flow causes a decrease in the diastolic blood pressure in the aorta, and therefore an increase in the pulse pressure (systolic pressure - diastolic pressure). Thus, physical examination will reveal a bounding pulse, especially in the radial artery. There is decreased effective forward flow in aortic insufficiency.
Note that while diastolic blood pressure is diminished and the pulse pressure widens, systolic blood pressure generally remains normal or can even be slightly elevated. This is because sympathetic nervous system and the renin-angiotensin-aldosterone axis of the kidneys compensate for the decreased cardiac output. Catecholamines will increase the heart rate and increase the strength of ventricular contraction, directly increasing cardiac output. Catecholamines will also cause peripheral vasoconstriction, which causes increased systemic vascular resistance and ensures that core organs are adequately perfused. Renin, a proteolytic enzyme, cleaves angiotensinogen to angiotensin I, which is converted to angiotensin II, which is also a potent vasoconstrictor. In the case of chronic aortic insufficiency with resultant cardiac remodeling, heart failure will develop, and it is possible to see systolic pressure diminishes.
Aortic insufficiency causes both volume overload (elevated preload) and pressure overload (elevated afterload due to increased stroke volume) of the heart. The pressure overload causes left ventricular hypertrophy (LVH). There is both concentric hypertrophy and eccentric hypertrophy in AI. The concentric hypertrophy is due to the increased left ventricular systolic pressures associated with AI, while the eccentric hypertrophy is due to volume overload caused by the regurgitant fraction.
Aortic Valve vs Aortic Root Causes
Aortic Valve Disease
A full list of causes of aortic insufficiency can be found on the page dedicated to the Differential Diagnosis of Underlying Causes.
One of the most common causes of aortic valvular disease in the past has been rheumatic fever in which case the aortic cusps are infiltrated with fibrous tissue. This then leads to retraction of the cusps and prevents their apposition during diastole. The cusps may also fuse and this may cause a component of aortic stenosis. It is therefore not uncommon for these patients to have mixed aortic regurgitation and aortic stenosis. Often these patients will have involvement of the mitral valve as well.
In the modern era, a more common cause of acquired aortic valve regurgitation is degenerative disease of the aorta and aortic valve in which case there is calcification and fibrosis of the cusps. As is the case with rheumatic fever, there is similar retraction of the cusps that results in aortic insufficiency.
A third not uncommon cause of acquired aortic regurgitation is infective endocarditis. In this disease state, regurgitation develops as a result of a hole or perforation that develops in the leaflet, or alternatively the cusps may not oppose each other due to a vegetation lying between the cusps which prevents their apposition.
Drugs such as dopamine agonists cause activation of serotonin -2B receptors (located in aortic valve and mitral valve) resulting in stimulation of fibroblast growth and fibrogenesis, thereby causing aortic insufficiency[1][2][3].
A final not uncommon cause of acquired aortic insufficiency is following a blunt chest trauma or a deceleration injury which causes traumatic aortic valve rupture resulting in distortion of the valve architecture leading to failure of the cusps to oppose[4][5].
Congenital conditions such as congenital bicuspid aortic stenosis or a ventricular septal defect can also result in aortic insufficiency. Patients with bicuspid aortic valve are at increased risk of developing aortic dissection[6].
Aortic Root Disease
Aortic root disease as a cause of aortic insufficiency has overtaken acquired forms of valvular disease and congenital forms of valvular disease as the leading cause of aortic regurgitation. The following is a list of those conditions that lead to dilation of the aortic root and thereby cause aortic insufficiency:
- Age-related degeneration,
- Hypertension
- Aortic dissection either causes sinus dilatation with incomplete leaflet coaptation at the center of the valve or dissection extending into the base of the leaflet with the resultant flial valve leaflet.
- Cystic medial necrosis of the aorta
- Giant cell arteritis
- Syphilitic aortitis
- Behçet's syndrome
- Ankylosing spondylitis
- Psoriatic arthritis
- Reiter's syndrome
- Ulcerative colitis
- Osteogenesis imperfecta
- Relapsing polychondritis
Acute vs Chronic Causes
Acute Aortic Insufficiency
- Rheumatic Fever
- Bacterial Endocarditis
- Aortic dissection
- Traumatic aortic rupture following blunt chest trauma
- After aortic balloon valvotomy[7]
- Myxomatous aortic valve
Chronic Aortic Insufficiency
- After aortic balloon valvotomy[7]
- Bicuspid aortic valve
- Aortic Dissection
- Hypertension
- Rheumatic Fever
- Bacterial Endocarditis
- Arteriosclerosis
- Myxomatous aortic valve
- Cystic medianecrosis of aorta
- Pseudoxanthoma Elasticum
- Ehlers-Danlos Syndrome
- Marfan Syndrome
- Bechterew's Disease
- Rheumatoid Arthritis
- Ankylosing Spondylitis
- Reiter's Syndrome
- Systemic Lupus Erythematosus
- Polymyalgia Rheumatica
- Turner's Syndrome
- Ventricular Septal Defect
- Sinus of Valsalva Aneurysm
- Syphilis
- Weight loss medications
Hemodynamic Consequences of Aortic Insufficiency
Acute aortic insufficiency
Acute aortic insufficiency is often secondary to infective endocarditis, aortic dissection[8] or traumatic aortic rupture[4][9].
In acute aortic insufficiency, there is sudden decrease in stroke volume secondary to regurgitation of blood into left ventricle with resultant sudden increase left ventricular end diastolic volume causing decreased stroke volume and cardiac output with subsequent cycles. The very high left ventricular end diastolic pressure and reflex tachycardia (due to decrease cardiac output) causes profound hypotension and cardiogenic shock.
Also the sharply rising left ventricular end diastolic pressure causes the mitral valve to close earlier during diastole. This early closure fortunately prevents backward flow of blood into the pulmonary vascular bed and often keeps the aortic diastolic pressure from falling too low and thus there is often not a wide pulse pressure. But in severe cases, the left ventricular end diastolic pressure equalizes with the aortic end-diastolic pressure leading to backward flow of blood causing elevation of left atrial and pulmonary venous pressures with resultant development of pulmonary edema. Indeed absence of a wide pulse pressure in the patient with acute aortic insufficiency should alert the clinician to potential left ventricular failure.
Chronic Aortic Insufficiency
In chronic aortic insufficiency, the aortic valve is unable to remain closed during diastole, which results in a portion of blood volume regurgitating into the left ventricle, thereby adding to the left ventricular end diastolic volume. Initially the heart adapts well to chronic aortic insufficiency as the left ventricle remains complaint. Therefore, the associated volume overload status is compensated by progressive left ventricular dilation and left ventricular hypertrophy, thereby increasing the stroke volume and cardiac output despite the regurgitant lesion.
In aortic insufficiency there is eccentric hypertrophy where in the sarcomers replicate in series and there is elongation of the myocytes and myocardial fibrils. As a result of this hypertrophy the ratio of the ventricular wall thickness to cavity radius remains normal and therefore wall stress is normal. In aortic stenosis there is concentric hypertrophy where in the sarcomers replicate in parallel.
Once wall thickening fails to keep up with the hemodynamic load, end systolic wall stress rises and at this point the left ventricle fails. The dramatic enlargement of the heart that is seen with aortic insufficiency is called cor bovinum. Over time the left ventricle will decompensate and there will be increasing interstitial fibrosis and stiffening causing reduction in the left ventricular wall compliance. At this point the patient will experience a rise in the left ventricular end diastolic volume and pressure. The first decline is seen with exercise and then the patient begins to have a reduction in forward output at rest.
Patients with chronic aortic insufficiency may also develop myocardial ischemia. This is due to the fact that they have an increase in demand due to an increased thickness of the left ventricle and also a reduction in the supply due to a lower perfusion pressure during diastole.
It has been said that 'aortic regurgitation begets aortic regurgitation'. The high oscillatory shear associated with aortic regurgitation may lead to further dilation of the aorta, which in turn may lead to further worsening of aortic regurgitation.
The mitral valve ring may also dilate leading to mitral regurgitation which further can progress to the development of left atrium dilatation.
References
- ↑ Waller EA, Kaplan J, Heckman MG (2005). "Valvular heart disease in patients taking pergolide". Mayo Clinic Proceedings. Mayo Clinic. 80 (8): 1016–20. PMID 16092580. Retrieved 2011-03-28. Unknown parameter
|month=ignored (help) - ↑ Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, Roth BL (2000). "Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications". Circulation. 102 (23): 2836–41. PMID 11104741. Retrieved 2011-03-28. Unknown parameter
|month=ignored (help) - ↑ Schade R, Andersohn F, Suissa S, Haverkamp W, Garbe E (2007). "Dopamine agonists and the risk of cardiac-valve regurgitation". The New England Journal of Medicine. 356 (1): 29–38. doi:10.1056/NEJMoa062222. PMID 17202453. Retrieved 2011-03-28. Unknown parameter
|month=ignored (help) - ↑ 4.0 4.1 Prêtre R, Faidutti B (1993). "Surgical management of aortic valve injury after nonpenetrating trauma". The Annals of Thoracic Surgery. 56 (6): 1426–31. PMID 8267458. Retrieved 2011-03-28. Unknown parameter
|month=ignored (help) - ↑ Onorati F, De Santo LS, Carozza A, De Feo M, Renzulli A, Cotrufo M (2004). "Marfan syndrome as a predisposing factor for traumatic aortic insufficiency". The Annals of Thoracic Surgery. 77 (6): 2192–4. doi:10.1016/S0003-4975(03)01409-7. PMID 15172299. Retrieved 2011-03-28. Unknown parameter
|month=ignored (help) - ↑ Fedak PW, Verma S, David TE, Leask RL, Weisel RD, Butany J (2002). "Clinical and pathophysiological implications of a bicuspid aortic valve". Circulation. 106 (8): 900–4. PMID 12186790. Retrieved 2011-03-28. Unknown parameter
|month=ignored (help) - ↑ 7.0 7.1 Isner JM (1991). "Acute catastrophic complications of balloon aortic valvuloplasty. The Mansfield Scientific Aortic Valvuloplasty Registry Investigators". Journal of the American College of Cardiology. 17 (6): 1436–44. PMID 2016464. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Roberts WC, Ko JM, Moore TR, Jones WH (2006). "Causes of pure aortic regurgitation in patients having isolated aortic valve replacement at a single US tertiary hospital (1993 to 2005)". Circulation. 114 (5): 422–9. doi:10.1161/CIRCULATIONAHA.106.622761. PMID 16864725. Retrieved 2011-03-28. Unknown parameter
|month=ignored (help) - ↑ Onorati F, De Santo LS, Carozza A, De Feo M, Renzulli A, Cotrufo M (2004). "Marfan syndrome as a predisposing factor for traumatic aortic insufficiency". The Annals of Thoracic Surgery. 77 (6): 2192–4. doi:10.1016/S0003-4975(03)01409-7. Retrieved 2011-03-28. Unknown parameter
|month=ignored (help)