Amlodipine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alonso Alvarado, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Amlodipine is a calcium channel blocker, dihydropirydine calcium channel blocker that is FDA approved for the treatment of hypertension, coronary artery disease. Common adverse reactions include flushing, palpitations, peripheral edema, abdominal pain, nausea, dizziness, headache, somnolence, fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Hypertension
- Dosing Information
- The usual initial antihypertensive oral dose of amlodipine is 5 mg once daily with a maximum dose of 10 mg once daily.
Small, fragile, or elderly patients, or patients with hepatic insufficiency may be started on 2.5 mg once daily and this dose may be used when adding amlodipine to other antihypertensive therapy.
Adjust dosage according to each patient's need. In general, titration should proceed over 7 to 14 days so that the physician can fully assess the patient's response to each dose level. Titration may proceed more rapidly, however, if clinically warranted, provided the patient is assessed frequently.
Coronary Artery Disease
- Dosing Information
- The recommended dose for chronic stable or vasospastic angina is 5–10 mg, with the lower dose suggested in the elderly and in patients with hepatic insufficiency. Most patients will require 10 mg for adequate effect [see Adverse Reactions].
The recommended dose range for patients with coronary artery disease is 5–10 mg once daily. In clinical studies, the majority of patients required 10 mg
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Amlodipine in adult patients.
Non–Guideline-Supported Use
Diabetic Nephropathy
- Dosing Information
Nondiabetic Kidney Disease
- Dosing Information
- 5-10 mg/day.[3]
Left Ventricular Hypertrophy
- Dosing Information
- Monotherapy: 5 mg/day, increase to 10 mg/day after first 14 days of treatment.
- Combination therapy: Amlodipine 5 mg/day for 14 days, then amlodipine 5 mg/day + benazepril 10 mg/day.[4]
Raynaud's Phenomenon
- Dosing Information
- 10 mg PO q24h.[5]
Silent Myocardial Ischemia
- Dosing Information
Systolic Hypertension
- Dosing Information
- 5 mg PO q24h.[9]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Hypertension
- Dosing Information
- The effective antihypertensive oral dose in pediatric patients ages 6–17 years is 2.5 mg to 5 mg once daily. Doses in excess of 5 mg daily have not been studied in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Amlodipine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Amlodipine in pediatric patients.
Contraindications
- Hypersensitivity to amlodipine.
Warnings
Hypotension
Symptomatic hypotension is possible, particularly in patients with severe aortic stenosis. Because of the gradual onset of action, acute hypotension is unlikely.
I==ncreased Angina or Myocardial Infarction==
Worsening angina and acute myocardial infarction can develop after starting or increasing the dose of amlodipine, particularly in patients with severe obstructive coronary artery disease.
Beta-Blocker Withdrawal
Amlodipine is not a beta-blocker and therefore gives no protection against the dangers of abrupt beta-blocker withdrawal; any such withdrawal should be by gradual reduction of the dose of beta-blocker.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
NORVASC has been evaluated for safety in more than 11,000 patients in U.S. and foreign clinical trials. In general, treatment with NORVASC was well-tolerated at doses up to 10 mg daily. Most adverse reactions reported during therapy with NORVASC were of mild or moderate severity. In controlled clinical trials directly comparing NORVASC (N=1730) at doses up to 10 mg to placebo (N=1250), discontinuation of NORVASC due to adverse reactions was required in only about 1.5% of patients and was not significantly different from placebo (about 1%). The most common side effects are headache and edema. The incidence (%) of side effects that occurred in a dose related manner are as follows:
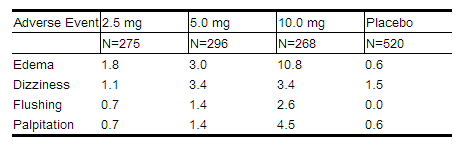
Other adverse experiences that were not clearly dose related but were reported with an incidence greater than 1.0% in placebo-controlled clinical trials include the following:
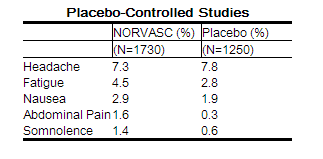
For several adverse experiences that appear to be drug and dose related, there was a greater incidence in women than men associated with amlodipine treatment as shown in the following table:
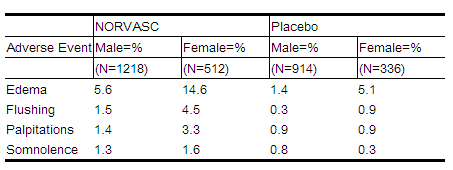
{clr
Postmarketing Experience
There is limited information regarding Amlodipine Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Amlodipine Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Amlodipine in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Amlodipine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Amlodipine during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Amlodipine in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Amlodipine in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Amlodipine in geriatric settings.
Gender
There is no FDA guidance on the use of Amlodipine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Amlodipine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Amlodipine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Amlodipine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Amlodipine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Amlodipine in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Amlodipine Administration in the drug label.
Monitoring
There is limited information regarding Amlodipine Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Amlodipine and IV administrations.
Overdosage
There is limited information regarding Amlodipine overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Amlodipine Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Amlodipine Mechanism of Action in the drug label.
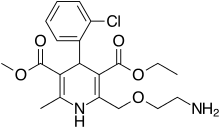
Structure
There is limited information regarding Amlodipine Structure in the drug label.
Pharmacodynamics
There is limited information regarding Amlodipine Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Amlodipine Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Amlodipine Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Amlodipine Clinical Studies in the drug label.
How Supplied
There is limited information regarding Amlodipine How Supplied in the drug label.
Storage
There is limited information regarding Amlodipine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Amlodipine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Amlodipine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Amlodipine Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Amlodipine interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Amlodipine Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Amlodipine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Seccia, T.M.; Vulpis, V.; Ricci, S.; Pirrelli, A. (1995). "Antihypertensive and Metabolic Effects of Amlodipine in Patients with Non-Insulin-Dependent Diabetes Mellitus". Clinical Drug Investigation. 9 (1): 16–21. doi:10.2165/00044011-199509010-00004. ISSN 1173-2563.
- ↑ Fogari R, Preti P, Zoppi A, Rinaldi A, Corradi L, Pasotti C; et al. (2002). "Effects of amlodipine fosinopril combination on microalbuminuria in hypertensive type 2 diabetic patients". Am J Hypertens. 15 (12): 1042–9. PMID 12460699.
- ↑ Esnault VL, Brown EA, Apetrei E, Bagon J, Calvo C, DeChatel R; et al. (2008). "The effects of amlodipine and enalapril on renal function in adults with hypertension and nondiabetic nephropathies: a 3-year, randomized, multicenter, double-blind, placebo-controlled study". Clin Ther. 30 (3): 482–98. doi:10.1016/j.clinthera.2008.03.006. PMID 18405787.
- ↑ Neutel JM, Smith DH, Weber MA (2004). "Effect of antihypertensive monotherapy and combination therapy on arterial distensibility and left ventricular mass". Am J Hypertens. 17 (1): 37–42. PMID 14700510.
- ↑ La Civita L, Pitaro N, Rossi M, Gambini I, Giuggioli D, Cini G; et al. (1993). "Amlodipine in the treatment of Raynaud's phenomenon". Br J Rheumatol. 32 (6): 524–5. PMID 8508292.
- ↑ Deanfield JE, Detry JM, Lichtlen PR, Magnani B, Sellier P, Thaulow E (1994). "Amlodipine reduces transient myocardial ischemia in patients with coronary artery disease: double-blind Circadian Anti-Ischemia Program in Europe (CAPE Trial)". J Am Coll Cardiol. 24 (6): 1460–7. PMID 7930276.
- ↑ Madjlessi-Simon T, Fillette F, Mary-Krause M, Lechat P, Jaillon P (1995). "Effects of amlodipine on transient myocardial ischaemia in patients with a severe coronary condition treated with a beta-blocker. Amlor-Holter Study Investigators". Eur Heart J. 16 (12): 1780–8. PMID 8682007.
- ↑ Bech J, Madsen JK, Kelbaek H (1999). "Amlodipine reduces myocardial ischaemia during exercise without compromising left ventricular function in patients with silent ischaemia: a randomised, double-blind, placebo-controlled study". Eur J Heart Fail. 1 (4): 395–400. PMID 10937953.
- ↑ Malacco E, Varì N, Capuano V, Spagnuolo V, Borgnino C, Palatini P; et al. (2003). "A randomized, double-blind, active-controlled, parallel-group comparison of valsartan and amlodipine in the treatment of isolated systolic hypertension in elderly patients: the Val-Syst study". Clin Ther. 25 (11): 2765–80. PMID 14693303.
The following events occurred in <1% but >0.1% of patients in controlled clinical trials or under conditions of open trials or marketing experience where a causal relationship is uncertain; they are listed to alert the physician to a possible relationship:
- Cardiovascular: Arrhythmia (including ventricular tachycardia and atrial fibrillation), bradycardia, chest pain, hypotension, peripheral ischemia, syncope, tachycardia, postural dizziness, postural hypotension, vasculitis.
- Central and Peripheral Nervous: Hypoesthesia, neuropathy peripheral, paresthesia, tremor, vertigo.
- Gastrointestinal: Anorexia, constipation, dyspepsia,1 dysphagia, diarrhea, flatulence, pancreatitis, vomiting, gingival hyperplasia.
- General: Allergic reaction, asthenia,2 back pain,hot flushes, malaise, pain, rigors, weight gain, weight decrease.
- Musculoskeletal System: Arthralgia, arthrosis, muscle cramps,3 myalgia.
- Psychiatric: Sexual dysfunction (male4 and female), insomnia, nervousness, depression, abnormal dreams, anxiety, depersonalization.
- Respiratory System: Dyspnea, epistaxis.
- Skin and Appendages: Angioedema, erythema multiforme, pruritus,6 rash,7 rash erythematous, rash maculopapular.
- Special Senses: Abnormal vision, conjunctivitis, diplopia, eye pain, tinnitus.
- Urinary System: Micturition frequency, micturition disorder, nocturia.
- Autonomic Nervous System: Dry mouth, sweating increased.
- Metabolic and Nutritional: Hyperglycemia, thirst.
- Hemopoietic: Leukopenia, purpura, thrombocytopenia.
Amlodipine therapy has not been associated with clinically significant changes in routine laboratory tests. No clinically relevant changes were noted in serum potassium, serum glucose, total triglycerides, total cholesterol, HDL cholesterol, uric acid, blood urea nitrogen, or creatinine.
In the CAMELOT and PREVENT studies, the adverse event profile was similar to that reported previously (see above), with the most common adverse event being peripheral edema.
|postmarketing=Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following postmarketing event has been reported infrequently where a causal relationship is uncertain: gynecomastia. In postmarketing experience, jaundice and hepatic enzyme elevations (mostly consistent with cholestasis or hepatitis), in some cases severe enough to require hospitalization, have been reported in association with use of amlodipine.
Amlodipine has been used safely in patients with chronic obstructive pulmonary disease, well-compensated congestive heart failure, coronary artery disease, peripheral vascular disease, diabetes mellitus, and abnormal lipid profiles. |drugInteractions=* Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description) |useInPregnancyFDA=(Description) |useInPregnancyAUS=(Description) |useInLaborDelivery=(Description) |useInNursing=(Description) |useInPed=(Description) |useInGeri=(Description) |useInGender=(Description) |useInRace=(Description) |useInHepaticImpair=Because amlodipine is extensively metabolized by the liver and the plasma elimination half-life (t 1/2) is 56 hours in patients with impaired hepatic function, titrate slowly when administering amlodipine to patients with severe hepatic impairment. |useInReproPotential=(Description) |useInImmunocomp=(Description) |othersTitle=Others |useInOthers=(Description) |administration=(Oral/Intravenous/etc) |monitoring======Condition 1=====
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section) |IVCompat====Solution===
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Y-Site
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Admixture
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Syringe
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
TPN/TNA
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
|overdose====Acute Overdose===
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description) |drugBox=
| |
Amlodipine
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
|mechAction=(Description) |structure=(Description with picture) |PD=(Description) |PK=(Description) |nonClinToxic=(Description) |clinicalStudies======Condition 1=====
(Description)
Condition 2
(Description)
Condition 3
(Description) |howSupplied=(Description) |fdaPatientInfo=(Patient Counseling Information) |alcohol=Alcohol-Amlodipine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. |lookAlike=* (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
|nlmPatientInfo=(Link to patient information page) |drugShortage=Drug Shortage }}