Chlorothiazide (injection)
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Nil |
| Elimination half-life | 45 to 120 minutes |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
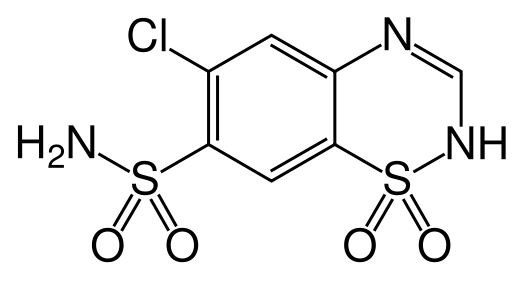
| Formula | C7H6ClN3O4S2 |
| Molar mass | 295.725 g/mol |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
For patient information, click here
Chlorothiazide sodium (Diuril) is a diuretic used within the hospital setting or for personal use to manage excess fluid associated with congestive heart failure. It is also used as an antihypertensive.
Most often taken in pill form, it is usually taken orally once or twice a day. In the ICU setting, chlorothiazide is given to diurese a patient in addition to furosemide (Lasix). Working in a separate mechanism than furosemide, and absorbed enterically as a reconstituted suspension administered through a nasogastric tube (NG tube), the two drugs potentiate one another without risk of toxicity. Because it is absorbed enterically there are no risks associated with chlorothiazide as there are with furosemide administration.
Indications
- Large amount of excess fluid including:
- Diagnosed congested heart failure
- Peripheral edema
- Rales / Rhonchi
- Hypertension
Contraindications
- Renal failure or insufficency
- Allergies to sulfa drugs
Dose
- 500 mg–1 g once or twice a day, by mouth or through NG tube (reconstituted suspension)
- May also be given intravenously, and should be given first if given in combination with IV lasix since it potentiate's the diuretic effect of furosemide.
Side effects
- Nausea / Vomiting
- Headache
- Dizziness
- Excess urine production
- Dehydration
- Hypoelectrolytemia (esp. hypokalemia / hypomagnesia)
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Thiazides
- Drugs