Rabies pathophysiology
|
Rabies Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Rabies pathophysiology On the Web |
|
American Roentgen Ray Society Images of Rabies pathophysiology |
|
Risk calculators and risk factors for Rabies pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
The rabies virus is categorized as a Lyssavirus. The molecular biology of rabies consists of bullet shaped virus with helical symmetry that has a length of approximately 180 nm. Rabies typically has its greatest effect on the brain. It is typically defined by encephalitis and myelitis. It is very important to avoid being bitten by a rabid animal because the virus is typically transmitted through the saliva of an infected organism.
Pathophysiology
The organism causing rabies is called Rabies virus (RV), a negative-stranded RNA virus of the rhabdovirus family. Rabies is an acute encephelomyelitis that causes disease in the human host via two features associated with the Rabies virus (RV):[1][2]
- Neurotropism
- Neuroinvasiveness
Transmission
- In Africa and Asia, domestic dogs are the main reservoirs of infection from Rabies virus[5]
- In the United States, racoons, foxes, skunks and bats rather than dogs spread the infection through bites[6]
Virology
The rabies virus is a Lyssavirus. This genus of RNA viruses also includes the Aravan virus, Australian bat lyssavirus, Duvenhage virus, European bat lyssavirus 1, European bat lyssavirus 2, Irkut virus, Khujand virus, Lagos bat virus, Mokola virus and West Caucasian bat virus. Lyssaviruses have helical symmetry, so their infectious particles are approximately cylindrical in shape. This is typical of plant-infecting viruses; human-infecting viruses more commonly have cubic symmetry and take shapes approximating regular polyhedra. Negri bodies in the infected neurons are pathognomonic.
- The virus has a bullet-like shape with a length of about 180 nm and a cross-sectional diameter of about 75 nm.
- One end is rounded or conical and the other end is planar or concave.
- The lipoprotein envelope carries knob-like spikes composed of Glycoprotein G. Spikes do not cover the planar end of the virion (virus particle).
- Beneath the envelope is the membrane or matrix (M) protein layer which may be invaginated at the planar end. The core of the virion consists of helically arranged ribonucleoprotein.
- The genome is unsegmented linear antisense RNA. Also present in the nucleocapsid are RNA dependent RNA transcriptase and some structural proteins.
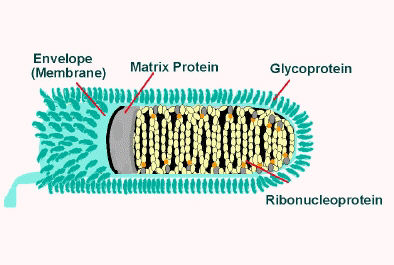
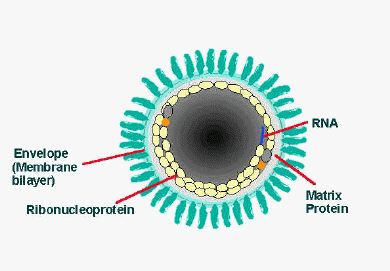
Longitudinal and cross-sectional schematic view of rabies virus
Transmission
The virus is usually present in the nerves and saliva of a symptomatic rabid animal.[7][8] The route of infection is usually, but not necessarily, by a bite. In many cases the affected animal is exceptionally aggressive, may attack without provocation, and exhibits otherwise uncharacteristic behaviour[2]. Transmission may also occur via an aerosol through mucous membranes; transmission in this form may have happened in people exploring caves populated by rabid bats. Transmission between humans is extremely rare, although it can happen through transplant surgery (see below for recent cases), or, even more rarely, through bites or kisses.
Transmission of rabies virus usually begins when infected saliva of a host is passed to an uninfected animal. Various routes of transmission have been documented and include contamination of mucous membranes (i.e., eyes, nose, mouth), aerosol transmission, and corneal transplantations. The most common mode of rabies virus transmission is through the bite and virus-containing saliva of an infected host.
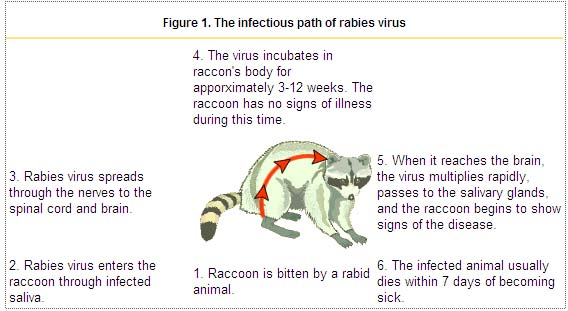
Following primary infection (see Figure, numbers 1 & 2), the virus enters an eclipse phase in which it cannot be easily detected within the host. This phase may last for several days or months. Investigations have shown both direct entry of virus into peripheral nerves at the site of infection and indirect entry after viral replication in non-nervous tissue (i.e., muscle cells). During the eclipse phase, the host immune defenses may confer cell-mediated immunity against viral infection because rabies virus is a good antigen. The uptake of virus into peripheral nerves is important for progressive infection to occur (see Figure, number 3).
After uptake into peripheral nerves, rabies virus is transported to the central nervous system (CNS) via retrograde axoplasmic flow. Typically this occurs via sensory and motor nerves at the initial site of infection. The incubation period (see figure, number 4) is the time from exposure to onset of clinical signs of disease. The incubation period may vary from a few days to several years, but is typically 1 to 3 months. Dissemination of virus within the CNS is rapid, and includes early involvement of limbic system neurons (see Figure, number 5). Active cerebral infection is followed by passive centrifugal spread of virus to peripheral nerves. The amplification of infection within the CNS occurs through cycles of viral replication and cell-to-cell transfer of progeny virus. Centrifugal spread of virus may lead to the invasion of highly innervated sites of various tissues, including the salivary glands. During this period of cerebral infection, the classic behavioral changes associated with rabies develop.
Rabies and Possums
Experimental studies of rabies infection in the Virginia opossum have shown the importance of the mode of transmission. Possums became infected when exposed to air-borne virus but were found to be fairly resistant to intramuscular inoculations [9][10][11].The aerosol transmission of rabies in opossum was investigated following the death from rabies of two men who had visited the Frio Caves, Texas, and did not remember any direct contact with bats.
Microscopic Pathology
Histologic examination of biopsy or autopsy tissues is occasionally useful in diagnosing unsuspected cases of rabies that have not been tested by routine methods. When brain tissue from rabies virus-infected animals are stained with a histologic stain, such as hematoxylin and eosin, evidence of encephalomyelitis may be recognized by a trained microscopist. This method is nonspecific and not considered diagnostic for rabies.
Before current diagnostic methods were available, rabies diagnosis was made using this method and the clinical case history. In fact, most of the significant histopathologic features (changes in tissue caused by disease) of rabies infection were described in the last quarter of the 19th century. After Louis Pasteur's successful experiments with rabies vaccination, scientists were motivated to identify the pathologic lesions of rabies virus.
Histopathologic evidence of rabies encephalomyelitis (inflammation) in brain tissue and meninges includes the following:
- Mononuclear infiltration
- Perivascular cuffing of lymphocytes or polymorphonuclear cells
- Lymphocytic foci
- Babes nodules consisting of glial cells
- Negri bodies
{{#ev:youtube|NP5CYphae5Y}}
Rabies and Dogs
Three stages of rabies are recognized in dogs. The first stage is a one to three day period characterized by behavioral changes and is known as the prodromal stage. The second stage is the excitative stage, which lasts three to four days. It is this stage that is often known as furious rabies due to the tendency of the affected dog to be hyperreactive to external stimuli and bite at anything near. The third stage is the paralytic stage and is caused by damage to motor neurons. Incoordination is seen due to rear limb paralysis and drooling and difficulty swallowing is caused by paralysis of facial and throat muscles. Death is usually caused by respiratory arrest.[12]
References
- ↑ Mrak RE, Young L (1994). "Rabies encephalitis in humans: pathology, pathogenesis and pathophysiology". J. Neuropathol. Exp. Neurol. 53 (1): 1–10. PMID 8301314.
- ↑ Lafon M (2004). "Subversive neuroinvasive strategy of rabies virus". Arch. Virol. Suppl. (18): 149–59. PMID 15119770.
- ↑ Swanepoel R, Barnard BJ, Meredith CD, Bishop GC, Brückner GK, Foggin CM, Hübschle OJ (1993). "Rabies in southern Africa". Onderstepoort J. Vet. Res. 60 (4): 325–46. PMID 7777317.
- ↑ Bingham J, Foggin CM, Wandeler AI, Hill FW (1999). "The epidemiology of rabies in Zimbabwe. 2. Rabies in jackals (Canis adustus and Canis mesomelas)". Onderstepoort J. Vet. Res. 66 (1): 11–23. PMID 10396757.
- ↑ "www.who.int" (PDF).
- ↑ "CDC - Rabies Surveillance in the U.S.: Wild Animals - Rabies".
- ↑ The Merck Manual, Eleventh Edition (1983), p. 183
- ↑ The Merck manual of Medical Information. Second Home Edition, (2003), p. 484.
- ↑ Constantine DG, Woodall DF. Related Articles, Links Transmission experiments with bat rabies isolates: reactions of certain Carnivora, possum, rodents, and bats to rabies virus of red bat origin when exposed by bat bite or by intrasmuscular inoculation. Am J Vet Res. 1966 Jan;27(116):24-32. No abstract available. PMID: 5913032 [PubMed - indexed for MEDLINE]
- ↑ Constantine DG 1967 Rabies transmission by air in bat caves. US Pub Health Serv, Publ. 1617
- ↑ 1: Am J Vet Res. 1960 May;21:507-10.Links Resistance of the opossum to rabies virus.BEAMER PD, MOHR CO, BARR TR. PMID: 13797881 [PubMed - indexed for MEDLINE]
- ↑ Ettinger, Stephen J.;Feldman, Edward C. (1995). Textbook of Veterinary Internal Medicine (4th ed. ed.). W.B. Saunders Company. ISBN 0-7216-6795-3.