Amlodipine adverse reactions: Difference between revisions
No edit summary |
No edit summary |
||
| Line 3: | Line 3: | ||
{{CMG}}; {{AE}} {{AK}} | {{CMG}}; {{AE}} {{AK}} | ||
== | == Adverse Reactions== | ||
=== | === Clinical Trials Experience=== | ||
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | ||
| Line 73: | Line 73: | ||
<sup>7</sup>These events occurred in less than 1% in placebo-controlled trials, but the incidence of these side effects was between 1% and 2% in all multiple dose studies. | <sup>7</sup>These events occurred in less than 1% in placebo-controlled trials, but the incidence of these side effects was between 1% and 2% in all multiple dose studies. | ||
=== | === Postmarketing Experience=== | ||
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | ||
Revision as of 01:12, 13 March 2014
| Amlodipine |
|---|
| NORVASC®, AMLODIPINE®, AMLODIPINE BESYLATE® FDA Package Insert |
| Indications and Usage |
| Dosage and Administration |
| Dosage Forms and Strengths |
| Contraindications |
| Warnings and Precautions |
| Adverse Reactions |
| Drug Interactions |
| Use in Specific Populations |
| Overdosage |
| Description |
| Clinical Pharmacology |
| Nonclinical Toxicology |
| Clinical Studies |
| How Supplied/Storage and Handling |
| Patient Counseling Information |
| Labels and Packages |
| Clinical Trials on Amlodipine |
| ClinicalTrials.gov |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
NORVASC has been evaluated for safety in more than 11,000 patients in U.S. and foreign clinical trials. In general, treatment with NORVASC was well-tolerated at doses up to 10 mg daily. Most adverse reactions reported during therapy with NORVASC were of mild or moderate severity. In controlled clinical trials directly comparing NORVASC (N=1730) at doses up to 10 mg to placebo (N=1250), discontinuation of NORVASC due to adverse reactions was required in only about 1.5% of patients and was not significantly different from placebo (about 1%). The most common side effects are headache and edema. The incidence (%) of side effects that occurred in a dose related manner are as follows:
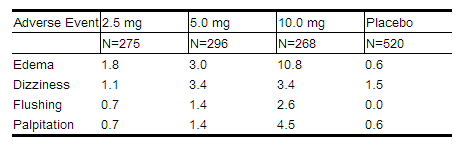 |
Other adverse experiences that were not clearly dose related but were reported with an incidence greater than 1.0% in placebo-controlled clinical trials include the following:
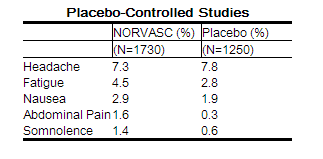 |
For several adverse experiences that appear to be drug and dose related, there was a greater incidence in women than men associated with amlodipine treatment as shown in the following table:
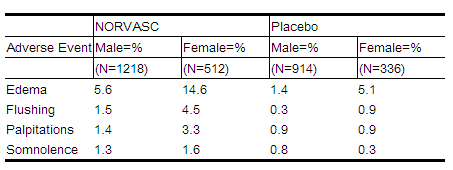 |
The following events occurred in <1% but >0.1% of patients in controlled clinical trials or under conditions of open trials or marketing experience where a causal relationship is uncertain; they are listed to alert the physician to a possible relationship:
Cardiovascular: arrhythmia (including ventricular tachycardia and atrial fibrillation), bradycardia, chest pain, hypotension, peripheral ischemia, syncope, tachycardia, postural dizziness, postural hypotension, vasculitis.
Central and Peripheral Nervous: hypoesthesia, neuropathy peripheral, paresthesia, tremor, vertigo.
Gastrointestinal: anorexia, constipation, dyspepsia,1 dysphagia, diarrhea, flatulence, pancreatitis, vomiting, gingival hyperplasia.
General: allergic reaction, asthenia,2 back pain,hot flushes, malaise, pain, rigors, weight gain, weight decrease.
Musculoskeletal System: arthralgia, arthrosis, muscle cramps,3 myalgia.
Psychiatric:sexual dysfunction (male4 and female), insomnia, nervousness, depression, abnormal dreams, anxiety, depersonalization.
Respiratory System: dyspnea,5 epistaxis.
Skin and Appendages: angioedema, erythema multiforme, pruritus,6 rash,7 rash erythematous, rash maculopapular.
Special Senses: abnormal vision, conjunctivitis, diplopia, eye pain, tinnitus.
Urinary System: micturition frequency, micturition disorder, nocturia.
Autonomic Nervous System: dry mouth, sweating increased.
Metabolic and Nutritional: hyperglycemia, thirst.
Hemopoietic: leukopenia, purpura, thrombocytopenia.
The following events occurred in <0.1% of patients: cardiac failure, pulse irregularity, extrasystoles, skin discoloration, urticaria, skin dryness, alopecia, dermatitis,muscle weakness, twitching, ataxia, hypertonia, migraine, cold and clammy skin, apathy, agitation, amnesia, gastritis, increased appetite, loose stools, coughing, rhinitis, dysuria, polyuria, parosmia, taste perversion, abnormal visual accommodation, and xerophthalmia.
Other reactions occurred sporadically and cannot be distinguished from medications or concurrent disease states such as myocardial infarction and angina.
NORVASC therapy has not been associated with clinically significant changes in routine laboratory tests. No clinically relevant changes were noted in serum potassium, serum glucose, total triglycerides, total cholesterol, HDL cholesterol, uric acid, blood urea nitrogen, or creatinine.
In the CAMELOT and PREVENT studies [see Clinical Studies (14.4)], the adverse event profile was similar to that reported previously (see above), with the most common adverse event being peripheral edema.
1These events occurred in less than 1% in placebo-controlled trials, but the incidence of these side effects was between 1% and 2% in all multiple dose studies.
2These events occurred in less than 1% in placebo-controlled trials, but the incidence of these side effects was between 1% and 2% in all multiple dose studies.
3These events occurred in less than 1% in placebo-controlled trials, but the incidence of these side effects was between 1% and 2% in all multiple dose studies.
4These events occurred in less than 1% in placebo-controlled trials, but the incidence of these side effects was between 1% and 2% in all multiple dose studies.
5These events occurred in less than 1% in placebo-controlled trials, but the incidence of these side effects was between 1% and 2% in all multiple dose studies.
6These events occurred in less than 1% in placebo-controlled trials, but the incidence of these side effects was between 1% and 2% in all multiple dose studies.
7These events occurred in less than 1% in placebo-controlled trials, but the incidence of these side effects was between 1% and 2% in all multiple dose studies.
Postmarketing Experience
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following postmarketing event has been reported infrequently where a causal relationship is uncertain: gynecomastia. In postmarketing experience, jaundice and hepatic enzyme elevations (mostly consistent with cholestasis or hepatitis), in some cases severe enough to require hospitalization, have been reported in association with use of amlodipine.
NORVASC has been used safely in patients with chronic obstructive pulmonary disease, well-compensated congestive heart failure, coronary artery disease, peripheral vascular disease, diabetes mellitus, and abnormal lipid profiles.[1]
References
- ↑ "NORVASC (AMLODIPINE BESYLATE) TABLET [CARDINAL HEALTH]". Retrieved 6 March 2014.