Fluticasone/salmeterol: Difference between revisions
No edit summary |
No edit summary |
||
| Line 233: | Line 233: | ||
=====Non–Potassium-Sparing Diuretics===== | =====Non–Potassium-Sparing Diuretics===== | ||
The [[ECG]] changes and/or [[hypokalemia]] that may result from the administration of non–potassium-sparing [[diuretics]] (such as [[loop diuretics]] or [[thiazide]] diuretics) can be acutely worsened by [[beta-agonists]], such as [[salmeterol]], a component of [[fluticasone]] + [[salmeterol]], especially when the recommended dose of the [[beta-agonist]] is exceeded. Although the clinical significance of these effects is not known, caution is advised in the coadministration of [[fluticasone]] + [[salmeterol]] with non–potassium‑sparing diuretics. | The [[ECG]] changes and/or [[hypokalemia]] that may result from the administration of non–potassium-sparing [[diuretics]] (such as [[loop diuretics]] or [[thiazide]] diuretics) can be acutely worsened by [[beta-agonists]], such as [[salmeterol]], a component of [[fluticasone]] + [[salmeterol]], especially when the recommended dose of the [[beta-agonist]] is exceeded. Although the clinical significance of these effects is not known, caution is advised in the coadministration of [[fluticasone]] + [[salmeterol]] with non–potassium‑sparing diuretics. | ||
|useInPregnancyFDA=( | |FDAPregCat=C | ||
|useInPregnancyFDA======Teratogenic Effects===== | |||
There are no adequate and well-controlled trials with [[fluticasone]] + [[salmeterol]] in pregnant women. [[Corticosteroids]] and beta2-agonists have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Because animal reproduction studies are not always predictive of human response, [[fluticasone]] + [[salmeterol]] should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Women should be advised to contact their physicians if they become pregnant while taking [[fluticasone]] + [[salmeterol]]. | |||
[[Fluticasone]] + [[salmeterol]]: In the mouse reproduction assay, [[fluticasone propionate]] by the subcutaneous route at a dose approximately 3/5 the maximum recommended human daily inhalation dose (MRHDID) (on a mg/m2 basis at a maternal subcutaneous dose of 150 mcg/kg/day) combined with oral [[salmeterol]] at a dose approximately 410 times the MRHDID (on a mg/m2 basis at a maternal oral dose of 10 mg/kg/day) produced cleft palate, fetal death, increased implantation loss, and delayed ossification. These observations are characteristic of glucocorticoids. No developmental toxicity was observed at combination doses of [[fluticasone propionate]] subcutaneously up to approximately 1/6 the MRHDID (on a mg/m2 basis at a maternal subcutaneous dose of 40 mcg/kg/day) and doses of [[salmeterol]] up to approximately 55 times the MRHDID (on a mg/m2 basis at a maternal oral dose of 1.4 mg/kg/day). In rats, combining [[fluticasone propionate]] subcutaneously at a dose equivalent to the MRHDID (on a mg/m2 basis at a maternal subcutaneous dose of 100 mcg/kg/day) and a dose of [[salmeterol]] at approximately 810 times the MRHDID (on a mg/m2 basis at a maternal oral dose of 10 mg/kg/day) produced decreased fetal weight, umbilical hernia, delayed ossification, and changes in the occipital bone. No such effects were seen when combining [[fluticasone propionate]] subcutaneously at a dose less than the MRHDID (on a mg/m2 basis at a maternal subcutaneous dose of 30 mcg/kg/day) and an oral dose of [[salmeterol]] at approximately 80 times the MRHDID (on a mg/m2 basis at a maternal oral dose of 1 mg/kg/day). | |||
| | |||
| | ======[[Fluticasone propionate]]====== | ||
| | Mice and rats at [[fluticasone propionate]] doses less than or equivalent to the MRHDID (on a mg/m2 basis at a maternal subcutaneous dose of 45 and 100 mcg/kg/day, respectively) showed fetal toxicity characteristic of potent [[corticosteroid]] compounds, including embryonic growth retardation, omphalocele, cleft palate, and retarded cranial ossification. No teratogenicity was seen in rats at doses approximately equivalent to the MRHDID (on a mg/m2 basis at maternal inhaled doses up to 68.7 mg/kg/day). | ||
In rabbits, fetal weight reduction and cleft palate were observed at a [[fluticasone propionate]] dose less than the MRHDID (on a mg/m2 basis at a maternal subcutaneous dose of 4 mcg/kg/day). However, no teratogenic effects were reported at [[fluticasone propionate]] doses up to approximately 5 times the MRHDID (on a mg/m2 basis at a maternal oral dose up to 300 mcg/kg/day). No [[fluticasone propionate]] was detected in the plasma in this study, consistent with the established low bioavailability following oral administration. | |||
| | [[Fluticasone propionate]] crossed the placenta following subcutaneous administration to mice and rats and oral administration to rabbits. | ||
| | Experience with oral [[corticosteroid]]s since their introduction in pharmacologic, as opposed to physiologic, doses suggests that rodents are more prone to teratogenic effects from [[corticosteroid]]s than humans. In addition, because there is a natural increase in [[corticosteroid]] production during pregnancy, most women will require a lower exogenous [[corticosteroid]] dose and many will not need [[corticosteroid]] treatment during pregnancy. | ||
| | |||
======Salmeterol====== | |||
No teratogenic effects occurred in rats at [[salmeterol]] doses approximately 160 times the MRHDID (on a mg/m2 basis at maternal oral doses up to 2 mg/kg/day). In pregnant Dutch rabbits administered [[salmeterol]] doses approximately 50 times the MRHDID (on an AUC basis at maternal oral doses of 1 mg/kg/day and higher), fetal toxic effects were observed characteristically resulting from beta-adrenoceptor stimulation. These included precocious eyelid openings, cleft palate, sternebral fusion, limb and paw flexures, and delayed ossification of the frontal cranial bones. No such effects occurred at a [[salmeterol]] dose approximately 20 times the MRHDID (on an AUC basis at a maternal oral dose of 0.6 mg/kg/day). | |||
New Zealand White rabbits were less sensitive since only delayed ossification of the frontal cranial bones was seen at a [[salmeterol]] dose approximately 1,600 times the MRHDID on a mg/m2 basis at a maternal oral dose of 10 mg/kg/day. [[Salmeterol]] xinafoate crossed the placenta following oral administration to mice and rats. | |||
=====Nonteratogenic Effects===== | |||
[[Hypoadrenalism]] may occur in infants born of mothers receiving [[corticosteroid]]s during pregnancy. Such infants should be carefully monitored. | |||
|useInLaborDelivery=There are no well-controlled human trials that have investigated effects of [[fluticasone]] + [[salmeterol]] on preterm labor or labor at term. Because of the potential for beta-agonist interference with uterine contractility, use of [[fluticasone]] + [[salmeterol]] during labor should be restricted to those patients in whom the benefits clearly outweigh the risks. | |||
|useInNursing=Plasma levels of [[salmeterol]], a component of [[fluticasone]] + [[salmeterol]], after inhaled therapeutic doses are very low. In rats, [[salmeterol]] xinafoate is excreted in the milk. There are no data from controlled trials on the use of [[salmeterol]] by nursing mothers. It is not known whether fluticasone propionate, a component of [[fluticasone]] + [[salmeterol]], is excreted in human breast milk. However, other [[corticosteroid]]s have been detected in human milk. Subcutaneous administration to lactating rats of tritiated fluticasone propionate resulted in measurable radioactivity in milk. | |||
Since there are no data from controlled trials on the use of [[fluticasone]] + [[salmeterol]] by nursing mothers, caution should be exercised when [[fluticasone]] + [[salmeterol]] is administered to a nursing woman. | |||
|useInPed=Use of [[fluticasone]] + [[salmeterol]] 100/50 in patients aged 4 to 11 years is supported by extrapolation of efficacy data from older subjects and by safety and efficacy data from a trial of [[fluticasone]] + [[salmeterol]] 100/50 in children with asthma aged 4 to 11 years. The safety and effectiveness of [[fluticasone]] + [[salmeterol]] in children with asthma younger than 4 years have not been established. | |||
Inhaled [[corticosteroid]]s, including fluticasone propionate, a component of [[fluticasone]] + [[salmeterol]], may cause a reduction in growth velocity in children and adolescents. The growth of pediatric patients receiving orally inhaled [[corticosteroid]]s, including [[fluticasone]] + [[salmeterol]], should be monitored. | |||
A 52-week placebo-controlled trial to assess the potential growth effects of fluticasone propionate inhalation powder at 50 and 100 mcg twice daily was conducted in the US in 325 prepubescent children (244 males and 81 females) aged 4 to 11 years. The mean growth velocities at 52 weeks observed in the intent-to-treat population were 6.32 cm/year in the placebo group (n = 76), 6.07 cm/year in the 50-mcg group (n = 98), and 5.66 cm/year in the 100‑mcg group (n = 89). An imbalance in the proportion of children entering puberty between groups and a higher dropout rate in the placebo group due to poorly controlled asthma may be confounding factors in interpreting these data. A separate subset analysis of children who remained prepubertal during the trial revealed growth rates at 52 weeks of 6.10 cm/year in the placebo group (n = 57), 5.91 cm/year in the 50‑mcg group (n = 74), and 5.67 cm/year in the 100‑mcg group (n = 79). In children aged 8.5 years, the mean age of children in this trial, the range for expected growth velocity is: boys – 3rd percentile = 3.8 cm/year, 50th percentile = 5.4 cm/year, and 97th percentile = 7.0 cm/year; girls – 3rd percentile = 4.2 cm/year, 50th percentile = 5.7 cm/year, and 97th percentile = 7.3 cm/year. The clinical relevance of these growth data is not certain. | |||
If a child or adolescent on any [[corticosteroid]] appears to have growth suppression, the possibility that he/she is particularly sensitive to this effect of [[corticosteroid]]s should be considered. The potential growth effects of prolonged treatment should be weighed against the clinical benefits obtained. To minimize the systemic effects of orally inhaled [[corticosteroid]]s, including [[fluticasone]] + [[salmeterol]], each patient should be titrated to the lowest strength that effectively controls his/her [[asthma]]. | |||
|useInGeri=Clinical trials of [[fluticasone]] + [[salmeterol]] for asthma did not include sufficient numbers of subjects aged 65 years and older to determine whether older subjects with [[asthma]] respond differently than younger subjects. | |||
Of the total number of subjects in clinical trials receiving [[fluticasone]] + [[salmeterol]] for [[COPD]], 1,621 were aged 65 years and older and 379 were aged 75 years and older. Subjects with [[COPD]] aged 65 years and older had a higher incidence of serious adverse events compared with subjects younger than 65 years. Although the distribution of adverse events was similar in the 2 age-groups, subjects older than 65 years experienced more severe events. In two 1-year trials, the excess risk of pneumonia that was seen in subjects treated with [[fluticasone]] + [[salmeterol]] compared with those treated with [[salmeterol]] was greater in subjects older than 65 years than in subjects younger than 65 years. As with other products containing [[beta2-agonists]], special caution should be observed when using [[fluticasone]] + [[salmeterol]] in geriatric patients who have concomitant cardiovascular disease that could be adversely affected by [[beta2-agonists]]. Based on available data for [[fluticasone]] + [[salmeterol]] or its active components, no adjustment of dosage of [[fluticasone]] + [[salmeterol]] in geriatric patients is warranted. | |||
No relationship between [[fluticasone propionate]] systemic exposure and age was observed in 57 subjects with [[COPD]] (aged 40 to 82 years) given 250 or 500 mcg twice daily. | |||
|useInRenalImpair=Formal pharmacokinetic studies using [[fluticasone]] + [[salmeterol]] have not been conducted in patients with [[renal impairment]]. | |||
|useInHepaticImpair=Formal pharmacokinetic studies using [[fluticasone]] + [[salmeterol]] have not been conducted in patients with hepatic impairment. However, since both [[fluticasone propionate]] and [[salmeterol]] are predominantly cleared by hepatic metabolism, impairment of liver function may lead to accumulation of [[fluticasone propionate]] and [[salmeterol]] in plasma. Therefore, patients with hepatic disease should be closely monitored. | |||
|administration=(Oral/Intravenous/etc) | |administration=(Oral/Intravenous/etc) | ||
|monitoring======Condition 1===== | |monitoring======Condition 1===== | ||
Revision as of 15:56, 4 August 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alonso Alvarado, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: ASTHMA-RELATED DEATH
See full prescribing information for complete Boxed Warning.
ASTHMA-RELATED DEATH: Long-acting beta2-adrenergic agonists (LABA), such as salmeterol, one of the active ingredients in fluticasone + salmeterol, increase the risk of asthma-related death. Data from a large placebo-controlled US trial that compared the safety of salmeterol with placebo added to usual asthma therapy showed an increase in asthma-related deaths in subjects receiving salmeterol (13 deaths out of 13,176 subjects treated for 28 weeks on salmeterol versus 3 deaths out of 13,179 subjects on placebo). Currently available data are inadequate to determine whether concurrent use of inhaled corticosteroids or other long-term asthma control drugs mitigates the increased risk of asthma-related death from LABA. Available data from controlled clinical trials suggest that LABA increase the risk of asthma-related hospitalization in pediatric and adolescent patients.
Therefore, when treating patients with asthma, physicians should only prescribe fluticasone + salmeterol for patients not adequately controlled on a long-term asthma control medication, such as an inhaled corticosteroid, or whose disease severity clearly warrants initiation of treatment with both an inhaled corticosteroid and a LABA. Once asthma control is achieved and maintained, assess the patient at regular intervals and step down therapy (e.g., discontinue fluticasone + salmeterol) if possible without loss of asthma control and maintain the patient on a long-term asthma control medication, such as an inhaled corticosteroid. Do not use fluticasone + salmeterol for patients whose asthma is adequately controlled on low- or medium-dose inhaled corticosteroids.
|
Overview
Fluticasone/salmeterol is a corticosteroid, beta2-adrenergic agonist that is FDA approved for the treatment of asthma, maintenance treatment of chronic obstructive pulmonary disease (COPD). There is a Black Box Warning for this drug as shown here. Common adverse reactions include nausea, oral candidiasis, musculoskeletal pain, dizziness, bronchitis, cough, difficulty speaking, hoarse, pharyngitis, throat irritation, upper respiratory tract infection, viral lower respiratory tract infection.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Fluticasone + salmeterol should be administered as 1 inhalation twice daily by the orally inhaled route only. After inhalation, the patient should rinse his/her mouth with water without swallowing to help reduce the risk of oropharyngeal candidiasis.
More frequent administration or a greater number of inhalations (more than 1 inhalation twice daily) of the prescribed strength of fluticasone + salmeterol is not recommended as some patients are more likely to experience adverse effects with higher doses of fluticasone + salmeterol. Patients using fluticasone + salmeterol should not use additional LABA for any reason.
If asthma symptoms arise in the period between doses, an inhaled, short-acting beta2-agonist should be taken for immediate relief.
Asthma: Adult and Adolescent Patients Aged 12 Years and Older
- For patients aged 12 years and older, the dosage is 1 inhalation twice daily, approximately 12 hours apart.
- The recommended starting dosages for fluticasone + salmeterol for patients aged 12 years and older are based upon patients’ asthma severity.
- The maximum recommended dosage is fluticasone + salmeterol 500/50 twice daily.
- Improvement in asthma control following inhaled administration of fluticasone + salmeterol can occur within 30 minutes of beginning treatment, although maximum benefit may not be achieved for 1 week or longer after starting treatment. Individual patients will experience a variable time to onset and degree of symptom relief.
- For patients who do not respond adequately to the starting dosage after 2 weeks of therapy, replacing the current strength of fluticasone + salmeterol with a higher strength may provide additional improvement in asthma control.
- If a previously effective dosage regimen fails to provide adequate improvement in asthma control, the therapeutic regimen should be reevaluated and additional therapeutic options (e.g., replacing the current strength of fluticasone + salmeterol with a higher strength, adding additional inhaled corticosteroid, initiating oral corticosteroids) should be considered.
Chronic Obstructive Pulmonary Disease
- The recommended dosage for patients with COPD is 1 inhalation of fluticasone + salmeterol 250/50 twice daily, approximately 12 hours apart.
- If shortness of breath occurs in the period between doses, an inhaled, short-acting beta2-agonist should be taken for immediate relief.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Fluticasone/salmeterol in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Fluticasone/salmeterol in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
=Asthma: Pediatric Patients Aged 4 to 11 Years
For patients with asthma aged 4 to 11 years who are not controlled on an inhaled corticosteroid, the dosage is 1 inhalation of fluticasone + salmeterol 100/50 twice daily, approximately 12 hours apart.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Fluticasone/salmeterol in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Fluticasone/salmeterol in pediatric patients.
Contraindications
The use of fluticasone + salmeterol is contraindicated in the following conditions:
- Primary treatment of status asthmaticus or other acute episodes of asthma or COPD where intensive measures are required.
- Severe hypersensitivity to milk proteins.
Warnings
|
WARNING: ASTHMA-RELATED DEATH
See full prescribing information for complete Boxed Warning.
ASTHMA-RELATED DEATH: Long-acting beta2-adrenergic agonists (LABA), such as salmeterol, one of the active ingredients in fluticasone + salmeterol, increase the risk of asthma-related death. Data from a large placebo-controlled US trial that compared the safety of salmeterol with placebo added to usual asthma therapy showed an increase in asthma-related deaths in subjects receiving salmeterol (13 deaths out of 13,176 subjects treated for 28 weeks on salmeterol versus 3 deaths out of 13,179 subjects on placebo). Currently available data are inadequate to determine whether concurrent use of inhaled corticosteroids or other long-term asthma control drugs mitigates the increased risk of asthma-related death from LABA. Available data from controlled clinical trials suggest that LABA increase the risk of asthma-related hospitalization in pediatric and adolescent patients.
Therefore, when treating patients with asthma, physicians should only prescribe fluticasone + salmeterol for patients not adequately controlled on a long-term asthma control medication, such as an inhaled corticosteroid, or whose disease severity clearly warrants initiation of treatment with both an inhaled corticosteroid and a LABA. Once asthma control is achieved and maintained, assess the patient at regular intervals and step down therapy (e.g., discontinue fluticasone + salmeterol) if possible without loss of asthma control and maintain the patient on a long-term asthma control medication, such as an inhaled corticosteroid. Do not use fluticasone + salmeterol for patients whose asthma is adequately controlled on low- or medium-dose inhaled corticosteroids.
|
Asthma-Related Death
LABA, such as salmeterol, one of the active ingredients in fluticasone + salmeterol, increase the risk of asthma-related death. Currently available data are inadequate to determine whether concurrent use of inhaled corticosteroids or other long-term asthma control drugs mitigates the increased risk of asthma-related death from LABA. Available data from controlled clinical trials suggest that LABA increase the risk of asthma-related hospitalization in pediatric and adolescent patients. Therefore, when treating patients with asthma, physicians should only prescribe fluticasone + salmeterol for patients not adequately controlled on a long-term asthma control medication, such as an inhaled corticosteroid, or whose disease severity clearly warrants initiation of treatment with both an inhaled corticosteroid and a LABA. Once asthma control is achieved and maintained, assess the patient at regular intervals and step down therapy (e.g., discontinue fluticasone + salmeterol) if possible without loss of asthma control and maintain the patient on a long-term asthma control medication, such as an inhaled corticosteroid. Do not use fluticasone + salmeterol for patients whose asthma is adequately controlled on low- or medium-dose inhaled corticosteroids.
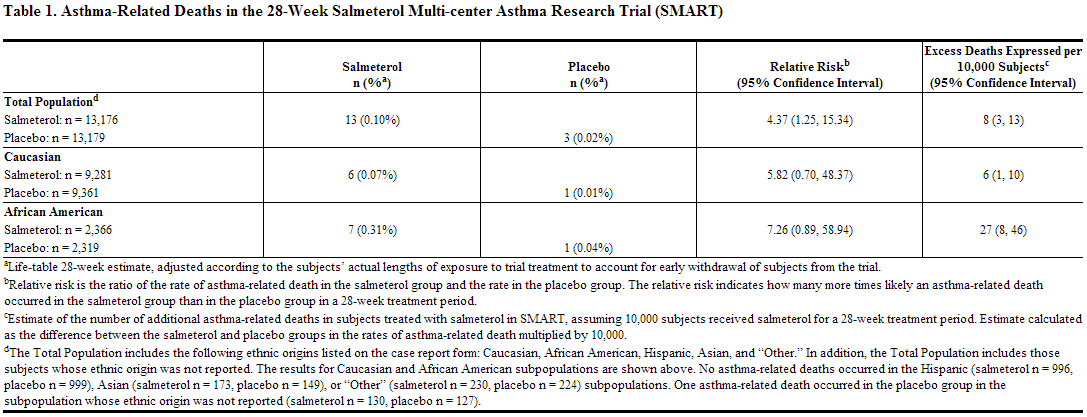
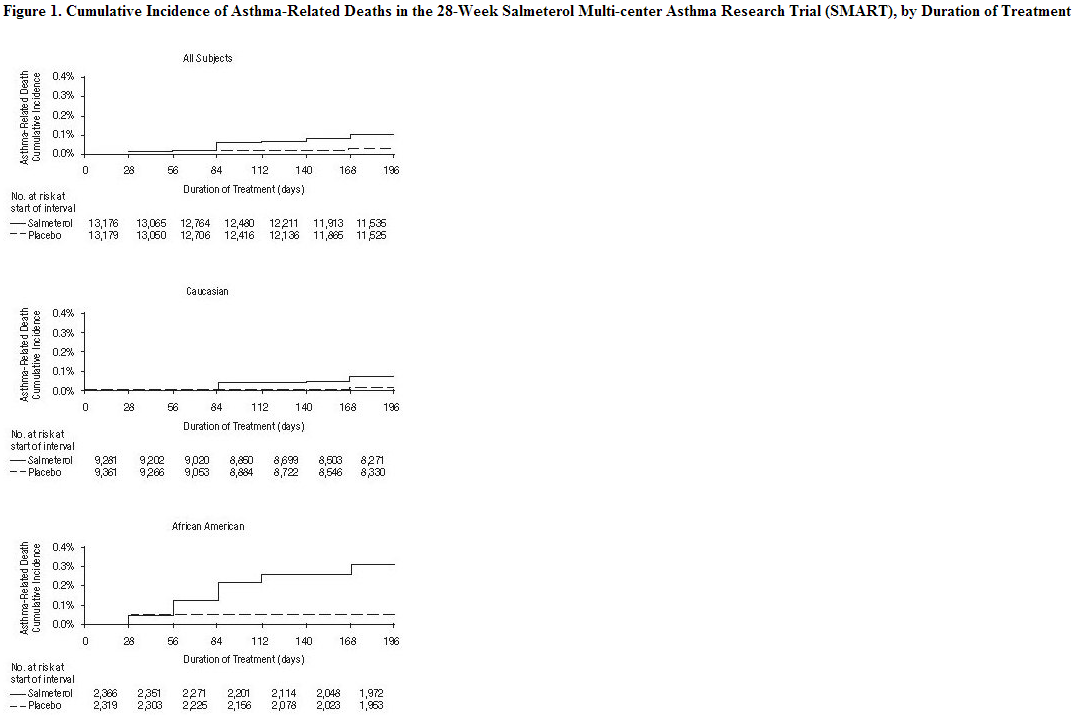
A large placebo‑controlled US trial that compared the safety of salmeterol with placebo, each added to usual asthma therapy, showed an increase in asthma-related deaths in subjects receiving salmeterol. The Salmeterol Multi-center Asthma Research Trial (SMART) was a randomized double-blind trial that enrolled LABA-naive subjects with asthma to assess the safety of salmeterol 42 mcg twice daily over 28 weeks compared with placebo when added to usual asthma therapy. A planned interim analysis was conducted when approximately half of the intended number of subjects had been enrolled (N = 26,355), which led to premature termination of the trial. The results of the interim analysis showed that subjects receiving salmeterol were at increased risk for fatal asthma events (see Table 1 and Figure 1). In the total population, a higher rate of asthma-related death occurred in subjects treated with salmeterol than those treated with placebo (0.10% versus 0.02%; relative risk: 4.37 [95% CI: 1.25, 15.34]). Post-hoc subpopulation analyses were performed. In Caucasians, asthma-related death occurred at a higher rate in subjects treated with salmeterol than in subjects treated with placebo (0.07% versus 0.01%; relative risk: 5.82 [95% CI: 0.70, 48.37]). In African Americans also, asthma‑related death occurred at a higher rate in subjects treated with salmeterol than those treated with placebo (0.31% versus 0.04%; relative risk: 7.26 [95% CI: 0.89, 58.94]). Although the relative risks of asthma‑related death were similar in Caucasians and African Americans, the estimate of excess deaths in subjects treated with salmeterol was greater in African Americans because there was a higher overall rate of asthma‑related death in African American subjects (see Table 1). Given the similar basic mechanisms of action of beta2‑agonists, the findings seen in the SMART trial are considered a class effect.
Post-hoc analyses in pediatric subjects aged 12 to 18 years were also performed. Pediatric subjects accounted for approximately 12% of subjects in each treatment arm. Respiratory-related death or life-threatening experience occurred at a similar rate in the salmeterol group (0.12% [2/1,653]) and the placebo group (0.12% [2/1,622]; relative risk: 1.0 [95% CI: 0.1, 7.2]). All-cause hospitalization, however, was increased in the salmeterol group (2% [35/1,653]) versus the placebo group (<1% [16/1,622]; relative risk: 2.1 [95% CI: 1.1, 3.7]).
The data from the SMART trial are not adequate to determine whether concurrent use of inhaled corticosteroids, such as fluticasone propionate, the other active ingredient in fluticasone + salmeterol, or other long-term asthma control therapy mitigates the risk of asthma-related death.
A 16-week clinical trial performed in the United Kingdom, the Salmeterol Nationwide Surveillance (SNS) trial, showed results similar to the SMART trial. In the SNS trial, the rate of asthma-related death was numerically, though not statistically significantly, greater in subjects with asthma treated with salmeterol (42 mcg twice daily) than those treated with albuterol (180 mcg 4 times daily) added to usual asthma therapy.
The SNS and SMART trials enrolled subjects with asthma. No trials have been conducted that were primarily designed to determine whether the rate of death in patients with COPD is increased by LABA.
Deterioration of Disease and Acute Episodes
Fluticasone + salmeterol should not be initiated in patients during rapidly deteriorating or potentially life-threatening episodes of asthma or COPD. Fluticasone + salmeterol has not been studied in subjects with acutely deteriorating asthma or COPD. The initiation of fluticasone + salmeterol in this setting is not appropriate.
Serious acute respiratory events, including fatalities, have been reported when salmeterol, a component of fluticasone + salmeterol, has been initiated in patients with significantly worsening or acutely deteriorating asthma. In most cases, these have occurred in patients with severe asthma (e.g., patients with a history of corticosteroid dependence, low pulmonary function, intubation, mechanical ventilation, frequent hospitalizations, previous life-threatening acute asthma exacerbations) and in some patients with acutely deteriorating asthma (e.g., patients with significantly increasing symptoms; increasing need for inhaled, short-acting beta2-agonists; decreasing response to usual medications; increasing need for systemic corticosteroids; recent emergency room visits; deteriorating lung function). However, these events have occurred in a few patients with less severe asthma as well. It was not possible from these reports to determine whether salmeterol contributed to these events.
Increasing use of inhaled, short-acting beta2-agonists is a marker of deteriorating asthma. In this situation, the patient requires immediate reevaluation with reassessment of the treatment regimen, giving special consideration to the possible need for replacing the current strength of fluticasone + salmeterol with a higher strength, adding additional inhaled corticosteroid, or initiating systemic corticosteroids. Patients should not use more than 1 inhalation twice daily of fluticasone + salmeterol.
Fluticasone + salmeterol should not be used for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm. An inhaled, short-acting beta2-agonist, not fluticasone + salmeterol, should be used to relieve acute symptoms such as shortness of breath. When prescribing fluticasone + salmeterol, the healthcare provider should also prescribe an inhaled, short-acting beta2-agonist (e.g., albuterol) for treatment of acute symptoms, despite regular twice-daily use of fluticasone + salmeterol.
When beginning treatment with fluticasone + salmeterol, patients who have been taking oral or inhaled, short-acting beta2-agonists on a regular basis (e.g., 4 times a day) should be instructed to discontinue the regular use of these drugs.
Excessive Use of Fluticasone + Salmeterol and Use With Other Long-Acting Beta2-Agonists
Fluticasone + salmeterol should not be used more often than recommended, at higher doses than recommended, or in conjunction with other medicines containing LABA, as an overdose may result. Clinically significant cardiovascular effects and fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs. Patients using fluticasone + salmeterol should not use another medicine containing a LABA (e.g., salmeterol, formoterol fumarate, arformoterol tartrate, indacaterol) for any reason.
Local Effects of Inhaled Corticosteroids
In clinical trials, the development of localized infections of the mouth and pharynx with Candida albicans has occurred in subjects treated with fluticasone + salmeterol. When such an infection develops, it should be treated with appropriate local or systemic (i.e., oral) antifungal therapy while treatment with fluticasone + salmeterol continues, but at times therapy with fluticasone + salmeterol may need to be interrupted. Advise the patient to rinse his/her mouth with water without swallowing following inhalation to help reduce the risk of oropharyngeal candidiasis.
Pneumonia
Physicians should remain vigilant for the possible development of pneumonia in patients with COPD as the clinical features of pneumonia and exacerbations frequently overlap.
Lower respiratory tract infections, including pneumonia, have been reported in patients with COPD following the inhaled administration of corticosteroids, including fluticasone propionate and fluticasone + salmeterol. In 2 replicate 1-year trials in 1,579 subjects with COPD, there was a higher incidence of pneumonia reported in subjects receiving fluticasone + salmeterol 250/50 (7%) than in those receiving salmeterol 50 mcg (3%). The incidence of pneumonia in the subjects treated with fluticasone + salmeterol was higher in subjects older than 65 years (9%) compared with the incidence in subjects younger than 65 years (4%).
In a 3-year trial in 6,184 subjects with COPD, there was a higher incidence of pneumonia reported in subjects receiving fluticasone + salmeterol 500/50 compared with placebo (16% with fluticasone + salmeterol 500/50, 14% with fluticasone propionate 500 mcg, 11% with salmeterol 50 mcg, and 9% with placebo). Similar to what was seen in the 1-year trials with fluticasone + salmeterol 250/50, the incidence of pneumonia was higher in subjects older than 65 years (18% with fluticasone + salmeterol 500/50 versus 10% with placebo) compared with subjects younger than 65 years (14% with fluticasone + salmeterol 500/50 versus 8% with placebo).
Inosuppression
Persons who are using drugs that suppress the immune system are more susceptible to infections than healthy individuals. Chickenpox and measles, for example, can have a more serious or even fatal course in susceptible children or adults using corticosteroids. In such children or adults who have not had these diseases or been properly immunized, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If a patient is exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If a patient is exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See the respective package inserts for complete VZIG and IG prescribing information.) If chickenpox develops, treatment with antiviral agents may be considered.
Inhaled corticosteroid should be used with caution, if at all, in patients with active or quiescent tuberculosis infections of the respiratory tract; systemic fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex.
Transferring Patients From Systemic Corticosteroid Therapy
Particular care is needed for patients who have been transferred from systemically active corticosteroids to inhaled corticosteroids because deaths due to adrenal insufficiency have occurred in patients with asthma during and after transfer from systemic corticosteroids to less systemically available inhaled corticosteroids. After withdrawal from systemic corticosteroids, a number of months are required for recovery of hypothalamic-pituitary-adrenal (HPA) function.
Patients who have been previously maintained on 20 mg or more of prednisone (or its equivalent) may be most susceptible, particularly when their systemic corticosteroids have been almost completely withdrawn. During this period of HPA suppression, patients may exhibit signs and symptoms of adrenal insufficiency when exposed to trauma, surgery, or infection (particularly gastroenteritis) or other conditions associated with severe electrolyte loss. Although fluticasone + salmeterol may control asthma symptoms during these episodes, in recommended doses it supplies less than normal physiological amounts of glucocorticoid systemically and does NOT provide the mineralocorticoid activity that is necessary for coping with these emergencies.
During periods of stress or a severe asthma attack, patients who have been withdrawn from systemic corticosteroids should be instructed to resume oral corticosteroids (in large doses) immediately and to contact their physicians for further instruction. These patients should also be instructed to carry a warning card indicating that they may need supplementary systemic corticosteroids during periods of stress or a severe asthma attack.
Patients requiring oral corticosteroids should be weaned slowly from systemic corticosteroid use after transferring to fluticasone + salmeterol. Prednisone reduction can be accomplished by reducing the daily prednisone dose by 2.5 mg on a weekly basis during therapy with fluticasone + salmeterol. Lung function (mean forced expiratory volume in 1 second [FEV1] or morning peak expiratory flow [AM PEF]), beta-agonist use, and asthma symptoms should be carefully monitored during withdrawal of oral corticosteroids. In addition, patients should be observed for signs and symptoms of adrenal insufficiency, such as fatigue, lassitude, weakness, nausea and vomiting, and hypotension.
Transfer of patients from systemic corticosteroid therapy to fluticasone + salmeterol may unmask allergic conditions previously suppressed by the systemic corticosteroid therapy (e.g., rhinitis, conjunctivitis, eczema, arthritis, eosinophilic conditions).
During withdrawal from oral corticosteroids, some patients may experience symptoms of systemically active corticosteroid withdrawal (e.g., joint and/or muscular pain, lassitude, depression) despite maintenance or even improvement of respiratory function.
Hypercorticism and Adrenal Suppression
Fluticasone propionate, a component of fluticasone + salmeterol, will often help control asthma symptoms with less suppression of HPA function than therapeutically equivalent oral doses of prednisone. Since fluticasone propionate is absorbed into the circulation and can be systemically active at higher doses, the beneficial effects of fluticasone + salmeterol in minimizing HPA dysfunction may be expected only when recommended dosages are not exceeded and individual patients are titrated to the lowest effective dose. A relationship between plasma levels of fluticasone propionate and inhibitory effects on stimulated cortisol production has been shown after 4 weeks of treatment with fluticasone propionate inhalation aerosol. Since individual sensitivity to effects on cortisol production exists, physicians should consider this information when prescribing fluticasone + salmeterol.
Because of the possibility of significant systemic absorption of inhaled corticosteroids in sensitive patients, patients treated with fluticasone + salmeterol should be observed carefully for any evidence of systemic corticosteroid effects. Particular care should be taken in observing patients postoperatively or during periods of stress for evidence of inadequate adrenal response.
It is possible that systemic corticosteroid effects such as hypercorticism and adrenal suppression (including adrenal crisis) may appear in a small number of patients who are sensitive to these effects. If such effects occur, fluticasone + salmeterol should be reduced slowly, consistent with accepted procedures for reducing systemic corticosteroids, and other treatments for management of asthma symptoms should be considered.
Drug Interactions With Strong Cytochrome P450 3A4 Inhibitors
The use of strong cytochrome P450 3A4 (CYP3A4) inhibitors (e.g., ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, ketoconazole, telithromycin) with fluticasone + salmeterol is not recommended because increased systemic corticosteroid and increased cardiovascular adverse effects may occur.
Paradoxical Bronchospasm and Upper Airway Symptoms
As with other inhaled medicines, fluticasone + salmeterol can produce paradoxical bronchospasm, which may be life threatening. If paradoxical bronchospasm occurs following dosing with fluticasone + salmeterol, it should be treated immediately with an inhaled, short-acting bronchodilator; fluticasone + salmeterol should be discontinued immediately; and alternative therapy should be instituted. Upper airway symptoms of laryngeal spasm, irritation, or swelling, such as stridor and choking, have been reported in patients receiving fluticasone + salmeterol.
Immediate Hypersensitivity Reactions
Immediate hypersensitivity reactions (e.g., urticaria, angioedema, rash, bronchospasm, hypotension), including anaphylaxis, may occur after administration of fluticasone + salmeterol. There have been reports of anaphylactic reactions in patients with severe milk protein allergy after inhalation of powder products containing lactose; therefore, patients with severe milk protein allergy should not use fluticasone + salmeterol.
Cardiovascular and Central Nervous System Effects
Excessive beta-adrenergic stimulation has been associated with seizures, angina, hypertension or hypotension, tachycardia with rates up to 200 beats/min, arrhythmias, nervousness, headache, tremor, palpitation, nausea, dizziness, fatigue, malaise, and insomnia. Therefore, fluticasone + salmeterol, like all products containing sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
Salmeterol, a component of fluticasone + salmeterol, can produce a clinically significant cardiovascular effect in some patients as measured by pulse rate, blood pressure, and/or symptoms. Although such effects are uncommon after administration of salmeterol at recommended doses, if they occur, the drug may need to be discontinued. In addition, beta-agonists have been reported to produce electrocardiogram (ECG) changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression. The clinical significance of these findings is unknown. Large doses of inhaled or oral salmeterol (12 to 20 times the recommended dose) have been associated with clinically significant prolongation of the QTc interval, which has the potential for producing ventricular arrhythmias. Fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs.
Reduction in Bone Mineral Density
Decreases in bone mineral density (BMD) have been observed with long-term administration of products containing inhaled corticosteroids. The clinical significance of small changes in BMD with regard to long-term consequences such as fracture is unknown. Patients with major risk factors for decreased bone mineral content, such as prolonged immobilization, family history of osteoporosis, postmenopausal status, tobacco use, advanced age, poor nutrition, or chronic use of drugs that can reduce bone mass (e.g., anticonvulsants, oral corticosteroids) should be monitored and treated with established standards of care. Since patients with COPD often have multiple risk factors for reduced BMD, assessment of BMD is recommended prior to initiating fluticasone + salmeterol and periodically thereafter. If significant reductions in BMD are seen and fluticasone + salmeterol is still considered medically important for that patient’s COPD therapy, use of medicine to treat or prevent osteoporosis should be strongly considered.
2-Year Fluticasone Propionate Trial: A 2-year trial in 160 subjects (females aged 18 to 40 years, males 18 to 50) with asthma receiving CFC-propelled fluticasone propionate inhalation aerosol 88 or 440 mcg twice daily demonstrated no statistically significant changes in BMD at any time point (24, 52, 76, and 104 weeks of double-blind treatment) as assessed by dual-energy x-ray absorptiometry at lumbar regions L1 through L4.
3-Year Bone Mineral Density Trial: Effects of treatment with fluticasone + salmeterol 250/50 or salmeterol 50 mcg on BMD at the L1-L4 lumbar spine and total hip were evaluated in 186 subjects with COPD (aged 43 to 87 years) in a 3-year double-blind trial. Of those enrolled, 108 subjects (72 males and 36 females) were followed for the entire 3 years. BMD evaluations were conducted at baseline and at 6-month intervals. Conclusions cannot be drawn from this trial regarding BMD decline in subjects treated with fluticasone + salmeterol versus salmeterol due to the inconsistency of treatment differences across gender and between lumbar spine and total hip.
In this trial there were 7 non-traumatic fractures reported in 5 subjects treated with fluticasone + salmeterol and 1 non-traumatic fracture in 1 subject treated with salmeterol. None of the non-traumatic fractures occurred in the vertebrae, hip, or long bones.
3-Year Survival Trial: Effects of treatment with fluticasone + salmeterol 500/50, fluticasone propionate 500 mcg, salmeterol 50 mcg, or placebo on BMD was evaluated in a subset of 658 subjects (females and males aged 40 to 80 years) with COPD in the 3-year survival trial. BMD evaluations were conducted at baseline and at 48, 108, and 158 weeks. Conclusions cannot be drawn from this trial because of the large number of dropouts (>50%) before the end of the follow-up and the maldistribution of covariates among the treatment groups that can affect BMD.
Fracture risk was estimated for the entire population of subjects with COPD in the survival trial (N = 6,184). The probability of a fracture over 3 years was 6.3% for fluticasone + salmeterol, 5.4% for fluticasone propionate, 5.1% for salmeterol, and 5.1% for placebo.
Effect on Growth
Orally inhaled corticosteroids may cause a reduction in growth velocity when administered to pediatric patients. Monitor the growth of pediatric patients receiving fluticasone + salmeterol routinely (e.g., via stadiometry). To minimize the systemic effects of orally inhaled corticosteroids, including fluticasone + salmeterol, titrate each patient’s dosage to the lowest dosage that effectively controls his/her symptoms.
Glaucoma and Cataracts
Glaucoma, increased intraocular pressure, and cataracts have been reported in patients with asthma and COPD following the long-term administration of inhaled corticosteroids, including fluticasone propionate, a component of fluticasone + salmeterol. Therefore, close monitoring is warranted in patients with a change in vision or with a history of increased intraocular pressure, glaucoma, and/or cataracts.
Effects of treatment with fluticasone + salmeterol 500/50, fluticasone propionate 500 mcg, salmeterol 50 mcg, or placebo on development of cataracts or glaucoma was evaluated in a subset of 658 subjects with COPD in the 3-year survival trial. Ophthalmic examinations were conducted at baseline and at 48, 108, and 158 weeks. Conclusions about cataracts cannot be drawn from this trial because the high incidence of cataracts at baseline (61% to 71%) resulted in an inadequate number of subjects treated with fluticasone + salmeterol 500/50 who were eligible and available for evaluation of cataracts at the end of the trial (n = 53). The incidence of newly diagnosed glaucoma was 2% with fluticasone + salmeterol 500/50, 5% with fluticasone propionate, 0% with salmeterol, and 2% with placebo.
Eosinophilic Conditions and Churg-Strauss Syndrome
In rare cases, patients on inhaled fluticasone propionate, a component of fluticasone + salmeterol, may present with systemic eosinophilic conditions. Some of these patients have clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition that is often treated with systemic corticosteroid therapy. These events usually, but not always, have been associated with the reduction and/or withdrawal of oral corticosteroid therapy following the introduction of fluticasone propionate. Cases of serious eosinophilic conditions have also been reported with other inhaled corticosteroids in this clinical setting. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients. A causal relationship between fluticasone propionate and these underlying conditions has not been established.
Coexisting Conditions
Fluticasone + salmeterol, like all medicines containing sympathomimetic amines, should be used with caution in patients with convulsive disorders or thyrotoxicosis and in those who are unusually responsive to sympathomimetic amines. Doses of the related beta2-adrenoceptor agonist albuterol, when administered intravenously, have been reported to aggravate preexisting diabetes mellitus and ketoacidosis.
Hypokalemia and Hyperglycemia
Beta-adrenergic agonist medicines may produce significant hypokalemia in some patients, possibly through intracellular shunting, which has the potential to produce adverse cardiovascular effects. The decrease in serum potassium is usually transient, not requiring supplementation. Clinically significant changes in blood glucose and/or serum potassium were seen infrequently during clinical trials with fluticasone + salmeterol at recommended doses.
Adverse Reactions
Clinical Trials Experience
LABA, such as salmeterol, one of the active ingredients in fluticasone + salmeterol, increase the risk of asthma-related death. Data from a large placebo-controlled US trial that compared the safety of salmeterol or placebo added to usual asthma therapy showed an increase in asthma-related deaths in subjects receiving salmeterol. Currently available data are inadequate to determine whether concurrent use of inhaled corticosteroids or other long-term asthma control drugs mitigates the increased risk of asthma-related death from LABA. Available data from controlled clinical trials suggest that LABA increase the risk of asthma-related hospitalization in pediatric and adolescent patients.
Systemic and local corticosteroid use may result in the following:
- Candida albicans infection.
- Pneumonia in patients with COPD.
- Immunosuppression.
- Hypercorticism and adrenal suppression.
- Reduction in bone mineral density.
- Growth effects.
- Glaucoma and cataracts.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trail Experience in Asthma
Adult and Adolescent Subjects Aged 12 Years and Older
The incidence of adverse reactions associated with fluticasone + salmeterol in Table 2 is based upon two 12‑week, placebo‑controlled, US clinical trials (Trials 1 and 2). A total of 705 adult and adolescent subjects (349 females and 356 males) previously treated with salmeterol or inhaled corticosteroids were treated twice daily with fluticasone + salmeterol (100/50- or 250/50-mcg doses), fluticasone propionate inhalation powder (100- or 250-mcg doses), salmeterol inhalation powder 50 mcg, or placebo. The average duration of exposure was 60 to 79 days in the active treatment groups compared with 42 days in the placebo group.
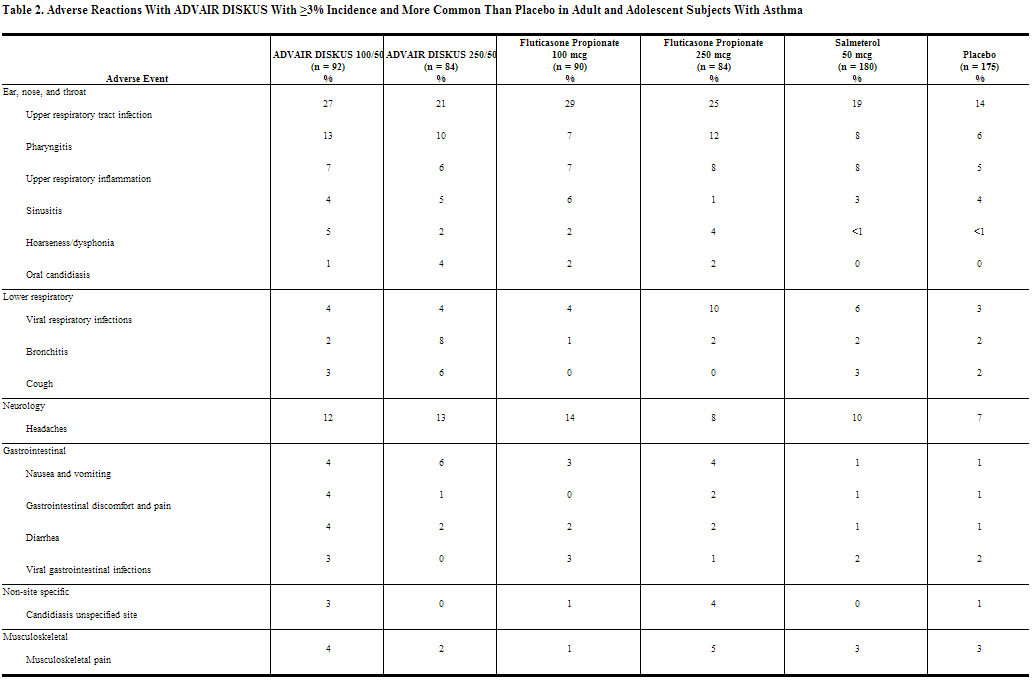
The types of adverse reactions and events reported in Trial 3, a 28-week non-US clinical trial in 503 subjects previously treated with inhaled corticosteroids who were treated twice daily with fluticasone + salmeterol 500/50, fluticasone propionate inhalation powder 500 mcg and salmeterol inhalation powder 50 mcg used concurrently, or fluticasone propionate inhalation powder 500 mcg, were similar to those reported in Table 2.
Additional Adverse Reactions
Other adverse reactions not previously listed, whether considered drug-related or not by the investigators, that were reported more frequently by subjects with asthma treated with fluticasone + salmeterol compared with subjects treated with placebo include the following: lymphatic signs and symptoms; muscle injuries; fractures; wounds and lacerations; contusions and hematomas; ear signs and symptoms; nasal signs and symptoms; nasal sinus disorders; keratitis and conjunctivitis; dental discomfort and pain; gastrointestinal signs and symptoms; oral ulcerations; oral discomfort and pain; lower respiratory signs and symptoms; pneumonia; muscle stiffness, tightness, and rigidity; bone and cartilage disorders; sleep disorders; compressed nerve syndromes; viral infections; pain; chest symptoms; fluid retention; bacterial infections; unusual taste; viral skin infections; skin flakiness and acquired ichthyosis; disorders of sweat and sebum.
Pediatric Subjects Aged 4 to 11 Years
The safety data for pediatric subjects aged 4 to 11 years is based upon 1 US trial of 12 weeks’ treatment duration. A total of 203 subjects (74 females and 129 males) who were receiving inhaled corticosteroids at trial entry were randomized to either fluticasone + salmeterol 100/50 or fluticasone propionate inhalation powder 100 mcg twice daily. Common adverse reactions (≥3% and greater than placebo) seen in the pediatric subjects but not reported in the adult and adolescent clinical trials include: throat irritation and ear, nose, and throat infections.
Laboratory Test Abnormalities
Elevation of hepatic enzymes was reported in ≥1% of subjects in clinical trials. The elevations were transient and did not lead to discontinuation from the trials. In addition, there were no clinically relevant changes noted in glucose or potassium.
Clinical Trials Experience in Chronic Obstructive Pulmonary Disease
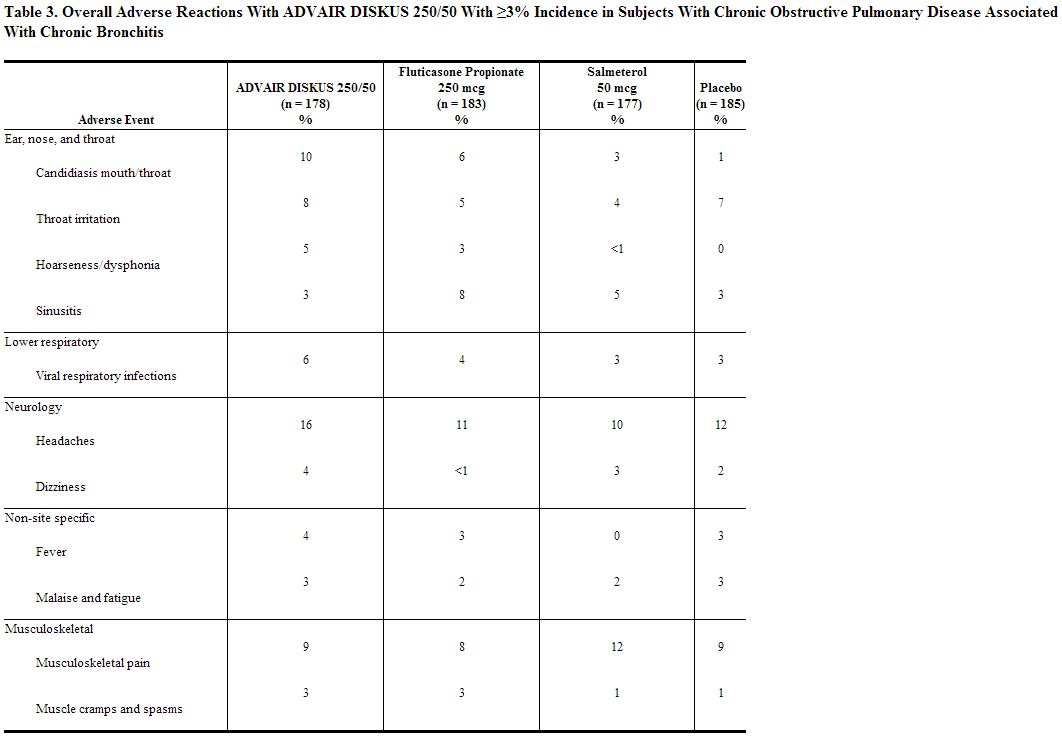
Short-Term (6 Months to 1 Year) Trials: The short-term safety data are based on exposure to fluticasone + salmeterol 250/50 twice daily in one 6-month and two 1-year clinical trials. In the 6-month trial, a total of 723 adult subjects (266 females and 457 males) were treated twice daily with fluticasone + salmeterol 250/50, fluticasone propionate inhalation powder 250 mcg, salmeterol inhalation powder, or placebo. The mean age of the subjects was 64, and the majority (93%) was Caucasian. In this trial, 70% of the subjects treated with fluticasone + salmeterol reported an adverse reaction compared with 64% on placebo. The average duration of exposure to fluticasone + salmeterol 250/50 was 141.3 days compared with 131.6 days for placebo. The incidence of adverse reactions in the 6-month trial is shown in Table 3.
In the two 1-year trials, fluticasone + salmeterol 250/50 was compared with salmeterol in 1,579 subjects (863 males and 716 females). The mean age of the subjects was 65 years, and the majority (94%) was Caucasian. To be enrolled, all of the subjects had to have had a COPD exacerbation in the previous 12 months. In this trial, 88% of the subjects treated with fluticasone + salmeterol and 86% of the subjects treated with salmeterol reported an adverse event. The most common events that occurred with a frequency of >5% and more frequently in the subjects treated with fluticasone + salmeterol were nasopharyngitis, upper respiratory tract infection, nasal congestion, back pain, sinusitis, dizziness, nausea, pneumonia, candidiasis, and dysphonia. Overall, 55 (7%) of the subjects treated with fluticasone + salmeterol and 25 (3%) of the subjects treated with salmeterol developed pneumonia.
The incidence of pneumonia was higher in subjects older than 65 years, 9% in the subjects treated with fluticasone + salmeterol compared with 4% in the subjects treated with fluticasone + salmeterol younger than 65 years. In the subjects treated with salmeterol, the incidence of pneumonia was the same (3%) in both age-groups.
Long-Term (3 Years) Trial: The safety of fluticasone + salmeterol 500/50 was evaluated in a randomized, double-blind, placebo-controlled, multicenter, international, 3-year trial in 6,184 adult subjects with COPD (4,684 males and 1,500 females). The mean age of the subjects was 65 years, and the majority (82%) was Caucasian. The distribution of adverse events was similar to that seen in the 1-year trials with fluticasone + salmeterol 250/50. In addition, pneumonia was reported in a significantly increased number of subjects treated with fluticasone + salmeterol 500/50 and fluticasone propionate 500 mcg (16% and 14%, respectively) compared with subjects treated with salmeterol 50 mcg or placebo (11% and 9%, respectively). When adjusted for time on treatment, the rates of pneumonia were 84 and 88 events per 1,000 treatment-years in the groups treated with fluticasone propionate 500 mcg and with fluticasone + salmeterol 500/50, respectively, compared with 52 events per 1,000 treatment-years in the salmeterol and placebo groups. Similar to what was seen in the 1-year trials with fluticasone + salmeterol 250/50, the incidence of pneumonia was higher in subjects older than 65 years (18% with fluticasone + salmeterol 500/50 versus 10% with placebo) compared with subjects younger than 65 years (14% with fluticasone + salmeterol 500/50 versus 8% with placebo).
Additional Adverse Reactions
Other adverse reactions not previously listed, whether considered drug-related or not by the investigators, that were reported more frequently by subjects with COPD treated with fluticasone + salmeterol compared with subjects treated with placebo include the following: syncope; ear, nose, and throat infections; ear signs and symptoms; laryngitis; nasal congestion/blockage; nasal sinus disorders; pharyngitis/throat infection; hypothyroidism; dry eyes; eye infections; gastrointestinal signs and symptoms; oral lesions; abnormal liver function tests; bacterial infections; edema and swelling; viral infections.
Laboratory Abnormalities
There were no clinically relevant changes in these trials. Specifically, no increased reporting of neutrophilia or changes in glucose or potassium was noted.
Postmarketing Experience
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, or causal connection to fluticasone + salmeterol, fluticasone propionate, and/or salmeterol or a combination of these factors.
- Cardiac Disorders: Arrhythmias (including atrial fibrillation, extrasystoles, supraventricular tachycardia), ventricular tachycardia.
- Endocrine Disorders: Cushing’s syndrome, Cushingoid features, growth velocity reduction in children/adolescents, hypercorticism.
- Eye Disorders: Glaucoma.
- Gastrointestinal Disorders: Abdominal pain, dyspepsia, xerostomia.
- Immune System Disorders: Immediate and delayed hypersensitivity reaction (including very rare anaphylactic reaction). Very rare anaphylactic reaction in patients with severe milk protein allergy.
- Infections and Infestations: Esophageal candidiasis.
- Metabolic and Nutrition Disorders: Hyperglycemia, weight gain.
- Musculoskeletal, Connective Tissue, and Bone Disorders: Arthralgia, cramps, myositis, osteoporosis.
- Nervous System Disorders: Paresthesia, restlessness.
- Psychiatric Disorders: Agitation, aggression, depression. Behavioral changes, including hyperactivity and irritability, have been reported very rarely and primarily in children.
- Reproductive System and Breast Disorders: Dysmenorrhea.
- Respiratory, Thoracic, and Mediastinal Disorders: Chest congestion; chest tightness; dyspnea; facial and oropharyngeal edema, immediate bronchospasm; paradoxical bronchospasm; tracheitis; wheezing; reports of upper respiratory symptoms of laryngeal spasm, irritation, or swelling such as stridor or choking.
- Skin and Subcutaneous Tissue Disorders: Ecchymoses, photodermatitis.
- Vascular Disorders: Pallor.
Drug Interactions
Fluticasone + salmeterol has been used concomitantly with other drugs, including short-acting beta2-agonists, methylxanthines, and intranasal corticosteroids, commonly used in patients with asthma or COPD without adverse drug reactions. No formal drug interaction trials have been performed with fluticasone + salmeterol.
Inhibitors of Cytochrome P450 3A4
Fluticasone propionate and salmeterol, the individual components of fluticasone + salmeterol, are substrates of CYP3A4. The use of strong CYP3A4 inhibitors (e.g., ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, ketoconazole, telithromycin) with fluticasone + salmeterol is not recommended because increased systemic corticosteroid and increased cardiovascular adverse effects may occur.
Ritonavir: Fluticasone Propionate: A drug interaction trial with fluticasone propionate aqueous nasal spray in healthy subjects has shown that ritonavir (a strong CYP3A4 inhibitor) can significantly increase plasma fluticasone propionate exposure, resulting in significantly reduced serum cortisol concentrations. During postmarketing use, there have been reports of clinically significant drug interactions in patients receiving fluticasone propionate and ritonavir, resulting in systemic corticosteroid effects including Cushing’s syndrome and adrenal suppression.
Ketoconazole: Fluticasone Propionate: Coadministration of orally inhaled fluticasone propionate (1,000 mcg) and ketoconazole (200 mg once daily) resulted in a 1.9-fold increase in plasma fluticasone propionate exposure and a 45% decrease in plasma cortisol area under the curve (AUC), but had no effect on urinary excretion of cortisol.
Salmeterol: In a drug interaction trial in 20 healthy subjects, coadministration of inhaled salmeterol (50 mcg twice daily) and oral ketoconazole (400 mg once daily) for 7 days resulted in greater systemic exposure to salmeterol (AUC increased 16-fold and Cmax increased 1.4-fold). Three (3) subjects were withdrawn due to beta2-agonist side effects (2 with prolonged QTc and 1 with palpitations and sinus tachycardia). Although there was no statistical effect on the mean QTc, coadministration of salmeterol and ketoconazole was associated with more frequent increases in QTc duration compared with salmeterol and placebo administration.
Monoamine Oxidase Inhibitors and Tricyclic Antidepressants
fluticasone + salmeterol should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors or tricyclic antidepressants, or within 2 weeks of discontinuation of such agents, because the action of salmeterol, a component of fluticasone + salmeterol, on the vascular system may be potentiated by these agents.
Beta-Adrenergic Receptor Blocking Agents
Beta-blockers not only block the pulmonary effect of beta-agonists, such as salmeterol, a component of fluticasone + salmeterol, but may also produce severe bronchospasm in patients with asthma or COPD. Therefore, patients with asthma or COPD should not normally be treated with beta-blockers. However, under certain circumstances, there may be no acceptable alternatives to the use of beta-blockers for these patients; cardioselective beta-blockers could be considered, although they should be administered with caution.
Non–Potassium-Sparing Diuretics
The ECG changes and/or hypokalemia that may result from the administration of non–potassium-sparing diuretics (such as loop diuretics or thiazide diuretics) can be acutely worsened by beta-agonists, such as salmeterol, a component of fluticasone + salmeterol, especially when the recommended dose of the beta-agonist is exceeded. Although the clinical significance of these effects is not known, caution is advised in the coadministration of fluticasone + salmeterol with non–potassium‑sparing diuretics.
Use in Specific Populations
Pregnancy
Teratogenic Effects
There are no adequate and well-controlled trials with fluticasone + salmeterol in pregnant women. Corticosteroids and beta2-agonists have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Because animal reproduction studies are not always predictive of human response, fluticasone + salmeterol should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Women should be advised to contact their physicians if they become pregnant while taking fluticasone + salmeterol.
Fluticasone + salmeterol: In the mouse reproduction assay, fluticasone propionate by the subcutaneous route at a dose approximately 3/5 the maximum recommended human daily inhalation dose (MRHDID) (on a mg/m2 basis at a maternal subcutaneous dose of 150 mcg/kg/day) combined with oral salmeterol at a dose approximately 410 times the MRHDID (on a mg/m2 basis at a maternal oral dose of 10 mg/kg/day) produced cleft palate, fetal death, increased implantation loss, and delayed ossification. These observations are characteristic of glucocorticoids. No developmental toxicity was observed at combination doses of fluticasone propionate subcutaneously up to approximately 1/6 the MRHDID (on a mg/m2 basis at a maternal subcutaneous dose of 40 mcg/kg/day) and doses of salmeterol up to approximately 55 times the MRHDID (on a mg/m2 basis at a maternal oral dose of 1.4 mg/kg/day). In rats, combining fluticasone propionate subcutaneously at a dose equivalent to the MRHDID (on a mg/m2 basis at a maternal subcutaneous dose of 100 mcg/kg/day) and a dose of salmeterol at approximately 810 times the MRHDID (on a mg/m2 basis at a maternal oral dose of 10 mg/kg/day) produced decreased fetal weight, umbilical hernia, delayed ossification, and changes in the occipital bone. No such effects were seen when combining fluticasone propionate subcutaneously at a dose less than the MRHDID (on a mg/m2 basis at a maternal subcutaneous dose of 30 mcg/kg/day) and an oral dose of salmeterol at approximately 80 times the MRHDID (on a mg/m2 basis at a maternal oral dose of 1 mg/kg/day).
Fluticasone propionate
Mice and rats at fluticasone propionate doses less than or equivalent to the MRHDID (on a mg/m2 basis at a maternal subcutaneous dose of 45 and 100 mcg/kg/day, respectively) showed fetal toxicity characteristic of potent corticosteroid compounds, including embryonic growth retardation, omphalocele, cleft palate, and retarded cranial ossification. No teratogenicity was seen in rats at doses approximately equivalent to the MRHDID (on a mg/m2 basis at maternal inhaled doses up to 68.7 mg/kg/day).
In rabbits, fetal weight reduction and cleft palate were observed at a fluticasone propionate dose less than the MRHDID (on a mg/m2 basis at a maternal subcutaneous dose of 4 mcg/kg/day). However, no teratogenic effects were reported at fluticasone propionate doses up to approximately 5 times the MRHDID (on a mg/m2 basis at a maternal oral dose up to 300 mcg/kg/day). No fluticasone propionate was detected in the plasma in this study, consistent with the established low bioavailability following oral administration.
Fluticasone propionate crossed the placenta following subcutaneous administration to mice and rats and oral administration to rabbits. Experience with oral corticosteroids since their introduction in pharmacologic, as opposed to physiologic, doses suggests that rodents are more prone to teratogenic effects from corticosteroids than humans. In addition, because there is a natural increase in corticosteroid production during pregnancy, most women will require a lower exogenous corticosteroid dose and many will not need corticosteroid treatment during pregnancy.
Salmeterol
No teratogenic effects occurred in rats at salmeterol doses approximately 160 times the MRHDID (on a mg/m2 basis at maternal oral doses up to 2 mg/kg/day). In pregnant Dutch rabbits administered salmeterol doses approximately 50 times the MRHDID (on an AUC basis at maternal oral doses of 1 mg/kg/day and higher), fetal toxic effects were observed characteristically resulting from beta-adrenoceptor stimulation. These included precocious eyelid openings, cleft palate, sternebral fusion, limb and paw flexures, and delayed ossification of the frontal cranial bones. No such effects occurred at a salmeterol dose approximately 20 times the MRHDID (on an AUC basis at a maternal oral dose of 0.6 mg/kg/day).
New Zealand White rabbits were less sensitive since only delayed ossification of the frontal cranial bones was seen at a salmeterol dose approximately 1,600 times the MRHDID on a mg/m2 basis at a maternal oral dose of 10 mg/kg/day. Salmeterol xinafoate crossed the placenta following oral administration to mice and rats.
Nonteratogenic Effects
Hypoadrenalism may occur in infants born of mothers receiving corticosteroids during pregnancy. Such infants should be carefully monitored.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Fluticasone/salmeterol in women who are pregnant.
Labor and Delivery
There are no well-controlled human trials that have investigated effects of fluticasone + salmeterol on preterm labor or labor at term. Because of the potential for beta-agonist interference with uterine contractility, use of fluticasone + salmeterol during labor should be restricted to those patients in whom the benefits clearly outweigh the risks.
Nursing Mothers
Plasma levels of salmeterol, a component of fluticasone + salmeterol, after inhaled therapeutic doses are very low. In rats, salmeterol xinafoate is excreted in the milk. There are no data from controlled trials on the use of salmeterol by nursing mothers. It is not known whether fluticasone propionate, a component of fluticasone + salmeterol, is excreted in human breast milk. However, other corticosteroids have been detected in human milk. Subcutaneous administration to lactating rats of tritiated fluticasone propionate resulted in measurable radioactivity in milk.
Since there are no data from controlled trials on the use of fluticasone + salmeterol by nursing mothers, caution should be exercised when fluticasone + salmeterol is administered to a nursing woman.
Pediatric Use
Use of fluticasone + salmeterol 100/50 in patients aged 4 to 11 years is supported by extrapolation of efficacy data from older subjects and by safety and efficacy data from a trial of fluticasone + salmeterol 100/50 in children with asthma aged 4 to 11 years. The safety and effectiveness of fluticasone + salmeterol in children with asthma younger than 4 years have not been established.
Inhaled corticosteroids, including fluticasone propionate, a component of fluticasone + salmeterol, may cause a reduction in growth velocity in children and adolescents. The growth of pediatric patients receiving orally inhaled corticosteroids, including fluticasone + salmeterol, should be monitored.
A 52-week placebo-controlled trial to assess the potential growth effects of fluticasone propionate inhalation powder at 50 and 100 mcg twice daily was conducted in the US in 325 prepubescent children (244 males and 81 females) aged 4 to 11 years. The mean growth velocities at 52 weeks observed in the intent-to-treat population were 6.32 cm/year in the placebo group (n = 76), 6.07 cm/year in the 50-mcg group (n = 98), and 5.66 cm/year in the 100‑mcg group (n = 89). An imbalance in the proportion of children entering puberty between groups and a higher dropout rate in the placebo group due to poorly controlled asthma may be confounding factors in interpreting these data. A separate subset analysis of children who remained prepubertal during the trial revealed growth rates at 52 weeks of 6.10 cm/year in the placebo group (n = 57), 5.91 cm/year in the 50‑mcg group (n = 74), and 5.67 cm/year in the 100‑mcg group (n = 79). In children aged 8.5 years, the mean age of children in this trial, the range for expected growth velocity is: boys – 3rd percentile = 3.8 cm/year, 50th percentile = 5.4 cm/year, and 97th percentile = 7.0 cm/year; girls – 3rd percentile = 4.2 cm/year, 50th percentile = 5.7 cm/year, and 97th percentile = 7.3 cm/year. The clinical relevance of these growth data is not certain.
If a child or adolescent on any corticosteroid appears to have growth suppression, the possibility that he/she is particularly sensitive to this effect of corticosteroids should be considered. The potential growth effects of prolonged treatment should be weighed against the clinical benefits obtained. To minimize the systemic effects of orally inhaled corticosteroids, including fluticasone + salmeterol, each patient should be titrated to the lowest strength that effectively controls his/her asthma.
Geriatic Use
Clinical trials of fluticasone + salmeterol for asthma did not include sufficient numbers of subjects aged 65 years and older to determine whether older subjects with asthma respond differently than younger subjects.
Of the total number of subjects in clinical trials receiving fluticasone + salmeterol for COPD, 1,621 were aged 65 years and older and 379 were aged 75 years and older. Subjects with COPD aged 65 years and older had a higher incidence of serious adverse events compared with subjects younger than 65 years. Although the distribution of adverse events was similar in the 2 age-groups, subjects older than 65 years experienced more severe events. In two 1-year trials, the excess risk of pneumonia that was seen in subjects treated with fluticasone + salmeterol compared with those treated with salmeterol was greater in subjects older than 65 years than in subjects younger than 65 years. As with other products containing beta2-agonists, special caution should be observed when using fluticasone + salmeterol in geriatric patients who have concomitant cardiovascular disease that could be adversely affected by beta2-agonists. Based on available data for fluticasone + salmeterol or its active components, no adjustment of dosage of fluticasone + salmeterol in geriatric patients is warranted.
No relationship between fluticasone propionate systemic exposure and age was observed in 57 subjects with COPD (aged 40 to 82 years) given 250 or 500 mcg twice daily.
Gender
There is no FDA guidance on the use of Fluticasone/salmeterol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Fluticasone/salmeterol with respect to specific racial populations.
Renal Impairment
Formal pharmacokinetic studies using fluticasone + salmeterol have not been conducted in patients with renal impairment.
Hepatic Impairment
Formal pharmacokinetic studies using fluticasone + salmeterol have not been conducted in patients with hepatic impairment. However, since both fluticasone propionate and salmeterol are predominantly cleared by hepatic metabolism, impairment of liver function may lead to accumulation of fluticasone propionate and salmeterol in plasma. Therefore, patients with hepatic disease should be closely monitored.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Fluticasone/salmeterol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Fluticasone/salmeterol in patients who are immunocompromised.
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
Solution
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Y-Site
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Admixture
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Syringe
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
TPN/TNA
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
| Error creating thumbnail: File missing | |
| |
Fluticasone/salmeterol
| |
| Combination of | |
| Fluticasone | Glucocorticoid |
| Salmeterol | Long-Acting Beta2 Agonist |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
P(UK) |
| Routes | ? |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
Storage
There is limited information regarding Fluticasone/salmeterol Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Fluticasone/salmeterol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Fluticasone/salmeterol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-Fluticasone/salmeterol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Fluticasone/salmeterol Brand Names in the drug label.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.