Diabetes mellitus type 1 pathophysiology: Difference between revisions
No edit summary |
No edit summary |
||
| (45 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Diabetes mellitus type 1}} | {{Diabetes mellitus type 1}} | ||
{{CMG}}; {{AE}} [[Priyamvada Singh|Priyamvada Singh, M.B.B.S.]] [mailto:psingh13579@gmail.com]; {{CZ}}{{VD}} {{Anahita}} | |||
==Overview== | ==Overview== | ||
[[Type 1 diabetes]] is a disorder characterized by abnormally high [[blood sugar]] levels. [[Type 1 diabetes]] is the result of interactions of [[Genetics|genetic]], [[Environmental Science|environmental]], and [[Immunology|immunologic]] factors that ultimately lead to the destruction of the pancreatic [[Beta cell|beta cells]] and [[insulin]] deficiency. Currently, 58 [[Genomics|genomic regions]] are known to be associated with [[Type 1 diabetes|type 1 DM]]. There are [[Environmental Science|environmental]] factors that can play a protective role in [[Type 1 diabetes]] like higher maternal [[vitamin D]], probiotic and [[omega-3 fatty acids]] intake during [[Obstetrics|prenatal]] period. Conversely, [[Environmental Science|environmental]] factors such as [[caesarean section]], [[Congenital rubella syndrome|congenital rubella]], maternal enteroviral infection and abnormal [[microbiome]] are among [[Environmental Science|environmental]] factors that are able to trigger [[Type 1 diabetes|Type 1 DM]]. Furthermore, some immunological components are responsible for [[Type 1 diabetes|type 1 DM]] pathogenesis. | |||
==Pathophysiology== | ==Pathophysiology== | ||
*[[Type 1 diabetes]] is a disorder characterized by abnormally high [[blood sugar]] levels. | |||
*In this form of [[diabetes]], specialized [[Cell (biology)|cells]] in the [[pancreas]] called [[Beta cells|beta cells]] stop producing [[insulin]]. [[Insulin]] controls how much [[glucose]] (a type of [[sugar]]) is passed from the [[blood]] into [[Cell (biology)|cells]] for conversion to [[energy]]. Lack of [[insulin]] results in the inability to use [[glucose]] for [[energy]] or to control the amount of [[sugar]] in the [[blood]]. | |||
*Possible [[thymus]] and [[bone marrow]] [[deficiency]] includes defective thymic selection, faulty self [[antigen]] presentation, thymic [[Variable number tandem repeat|VNTR]] and Aire [[Gene expression|expression]], mobilopathy and defective [[lymphocyte]] precursors are some possible proceeding triggers that underlie [[Diabetes Mellitus|type 1 DM]].<ref name="pmid23890997">{{cite journal| author=Atkinson MA, Eisenbarth GS, Michels AW| title=Type 1 diabetes. | journal=Lancet | year= 2014 | volume= 383 | issue= 9911 | pages= 69-82 | pmid=23890997 | doi=10.1016/S0140-6736(13)60591-7 | pmc=4380133 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23890997 }} </ref> | |||
===Pathogenesis=== | |||
*[[Diabetes Mellitus|Type 1 DM]] is the result of interactions of [[Genetics|genetic]], [[Environmental Science|environmental]], and [[Immunology|immunologic]] factors that ultimately lead to the destruction of the [[pancreas|pancreatic]] [[beta cells]] and [[insulin]] deficiency. | |||
*Concordance of [[Diabetes Mellitus|type 1 DM]] in identical [[twins]] ranges between 40 and 60%, indicating the presence of additional modifying factors.<br><br> | |||
[[File:Type-1-diabetes pathophysiology.jpg|600px|Type-1-diabetes pathophysiology|center]]<br><br> | |||
===Genetics=== | |||
*[[Genes]] associated with [[Diabetes mellitus]] include the following: <ref name="pmid27302272">{{cite journal| author=Pociot F, Lernmark Å| title=Genetic risk factors for type 1 diabetes. | journal=Lancet | year= 2016 | volume= 387 | issue= 10035 | pages= 2331-9 | pmid=27302272 | doi=10.1016/S0140-6736(16)30582-7 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27302272 }}</ref><ref name="pmid27625010">{{cite journal| author=Safari-Alighiarloo N, Taghizadeh M, Tabatabaei SM, Shahsavari S, Namaki S, Khodakarim S et al.| title=Identification of new key genes for type 1 diabetes through construction and analysis of protein-protein interaction networks based on blood and pancreatic islet transcriptomes. | journal=J Diabetes | year= 2016 | volume= | issue= | pages= | pmid=27625010 | doi=10.1111/1753-0407.12483 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27625010 }}</ref><ref>Brorsson CA, Pociot F, Type 1 Diabetes Genetics Consortium. Shared genetic basis for type 1 diabetes, islet autoantibodies, and autoantibodies associated with other immune-mediated diseases in families with type 1 diabetes. Diabetes Care 2015; 38 (suppl 3): S8–13.</ref><ref>Ahlqvist E, van Zuydam NR, Groop LC, McCarthy MI. The genetics of diabetic complications. Nat Rev Nephrol 2015; 11: 277–87.</ref><ref>Parkes M, Cortes A, van Heel DA, Brown MA. Genetic insights into common pathways and complex relationships among immune-mediated diseases. Nat Rev Genet 2013; 14: 661–73.</ref><ref name=":0">Type 1 Diabetes mellitus "Dennis Kasper, Anthony Fauci, Stephen Hauser, Dan Longo, J. Larry Jameson, Joseph Loscalzo"Harrison's Principles of Internal Medicine, 19e Accessed on December 27th,2016</ref> | |||
** Currently, 58 [[Genomics|genomic regions]] are known to be associated with [[Type 1 diabetes|Type 1 DM]]. | |||
** Major susceptibility [[gene]] for [[Type 1 diabetes|type 1 diabetes]] is located on [[HLA]] region of [[Chromosome 6 (human)|chromosome 6]]. It accounts for 40-50% of the genetic risk for [[Type 1 diabetes|type 1 diabetes]]. This region encodes for class II [[major histocompatibility complex]] ([[major histocompatibility complex|MHC]]) [[molecule|molecules]]. [[major histocompatibility complex]] ([[major histocompatibility complex|MHC]]) [[molecule|molecules]] play an important role in presenting [[antigen]] to [[T helper cell]] and initiating immune response. | |||
** Other major susceptibility [[genes]] which were associated with [[Type 1 diabetes|Type 1 DM]] include [[polymorphisms]] in the [[promoter region]] of the [[insulin]] [[gene]], the [[CTLA-4|CTLA-4 gene]], [[IL-2|interleukin 2 receptor]], [[Insulin]]-[[Variable number tandem repeat|VNTR]], AIRE, [[FOXP3|FoxP3]], [[STAT3]], HIP14 and [[PTPN22]] etc.<ref name="PaschouPapadopoulou-Marketou2018">{{cite journal|last1=Paschou|first1=Stavroula A|last2=Papadopoulou-Marketou|first2=Nektaria|last3=Chrousos|first3=George P|last4=Kanaka-Gantenbein|first4=Christina|title=On type 1 diabetes mellitus pathogenesis|journal=Endocrine Connections|volume=7|issue=1|year=2018|pages=R38–R46|issn=2049-3614|doi=10.1530/EC-17-0347}}</ref><ref name="Tuomi2005">{{cite journal|last1=Tuomi|first1=T.|title=Type 1 and Type 2 Diabetes: What Do They Have in Common?|journal=Diabetes|volume=54|issue=Supplement 2|year=2005|pages=S40–S45|issn=0012-1797|doi=10.2337/diabetes.54.suppl_2.S40}}</ref> | |||
** Presence of certain [[genes]] confer protection against the development of the disease. [[Haplotype]] DQA1*0102, DQB1*0602 is extremely rare in individuals with [[Type 1 diabetes|type 1 DM]] (<1%) and appears to provide protection from [[Type 1 diabetes|type 1 diabetes]]. | |||
** There is a relationship between some [[human leukocyte antigen|human leukocyte antigens]] ([[human leukocyte antigen|HLA]]) and [[Type 1 diabetes|type 1 diabetes]], such as DQB1, DQA1, and DRB1. There are some supporting data on DR4-linked haplotypes transmission from [[Diabetes mellitus type 2|type 2 diabetes]] parents to offspring with [[Type 1 diabetes|type 1 diabetes]]. Patients with latent autoimmune diabetes of adults also have been related to [[human leukocyte antigen|HLA]] alleles DQB1*0302 and 02. <ref name="Tuomi2005">{{cite journal|last1=Tuomi|first1=T.|title=Type 1 and Type 2 Diabetes: What Do They Have in Common?|journal=Diabetes|volume=54|issue=Supplement 2|year=2005|pages=S40–S45|issn=0012-1797|doi=10.2337/diabetes.54.suppl_2.S40}}</ref> | |||
<SMALL><SMALL> | |||
{| class="wikitable" | |||
![[Genes]] important to [[Type 1 diabetes|type 1 diabetes]] [[pathogenesis]] | |||
!Region | |||
!Odds ratio | |||
!Gene funtion | |||
|- | |||
| colspan="1" rowspan="1" |PTPN22 | |||
| colspan="1" rowspan="1" |1p13.2 | |||
| colspan="1" rowspan="1" |1·89 | |||
| colspan="1" rowspan="1" |Regulation of innate immune response, [[T cell]] activation, and [[natural killer cell]] [[Cell growth|proliferation]] | |||
|- | |||
| colspan="1" rowspan="1" |IL10 | |||
| colspan="1" rowspan="1" |1q32.1 | |||
| colspan="1" rowspan="1" |0·86 | |||
| colspan="1" rowspan="1" |[[Cytokine|Cytokines]] and inflammatory response | |||
|- | |||
| colspan="1" rowspan="1" |AFF3 | |||
| colspan="1" rowspan="1" |2q11.2 | |||
| colspan="1" rowspan="1" |1·11 | |||
| colspan="1" rowspan="1" |Regulation of [[Transcription (genetics)|transcription]] | |||
|- | |||
| colspan="1" rowspan="1" |IFIH1 | |||
| colspan="1" rowspan="1" |2q24.2 | |||
| colspan="1" rowspan="1" |0·85 | |||
0·85 | |||
0·59 | |||
| colspan="1" rowspan="1" |[[Innate immune system]] [[NF-κB]] activation | |||
|- | |||
| colspan="1" rowspan="1" |STAT4 | |||
| colspan="1" rowspan="1" |2q32.3 | |||
| colspan="1" rowspan="1" |1·10§ | |||
| colspan="1" rowspan="1" |[[Cytokine]]-mediated signalling pathway | |||
|- | |||
| colspan="1" rowspan="1" |CTLA4 | |||
| colspan="1" rowspan="1" |2q33.2 | |||
| colspan="1" rowspan="1" |0·82 | |||
0·84 | |||
| colspan="1" rowspan="1" |[[T cell]] activation | |||
|- | |||
| colspan="1" rowspan="1" |CCR5 | |||
| colspan="1" rowspan="1" |3p21.31 | |||
| colspan="1" rowspan="1" |0·85 | |||
| colspan="1" rowspan="1" |[[T helper cell|Th1 cell]] development and [[chemokine]]-mediated signalling pathway | |||
|- | |||
| colspan="1" rowspan="1" |IL21, IL2 | |||
| colspan="1" rowspan="1" |4q27 | |||
| colspan="1" rowspan="1" |1·13 | |||
1·12 | |||
1·14 | |||
1·15 | |||
| colspan="1" rowspan="1" |[[cytokine|Cytokines]] and inflammatory response and [[T helper cell|Th1 cell]] or [[T helper cell|Th2 cell]] [[differentiation]] | |||
|- | |||
| colspan="1" rowspan="1" |IL7R | |||
| colspan="1" rowspan="1" |5p13.2 | |||
| colspan="1" rowspan="1" |1·11 | |||
| colspan="1" rowspan="1" |[[T cell]]-mediated [[cytotoxicity]], [[immunoglobulin]] production, and [[antigen]] binding | |||
|- | |||
| colspan="1" rowspan="1" |BACH2 | |||
| colspan="1" rowspan="1" |6q15 | |||
| colspan="1" rowspan="1" |1·10 | |||
0·88 | |||
1·20 | |||
| colspan="1" rowspan="1" |[[Transcription (genetics)|transcription]] | |||
|- | |||
| colspan="1" rowspan="1" |TNFAIP3 | |||
| colspan="1" rowspan="1" |6q23.3 | |||
| colspan="1" rowspan="1" |1·12 | |||
| colspan="1" rowspan="1" |Inflammatory response | |||
|- | |||
| colspan="1" rowspan="1" |TAGAP | |||
| colspan="1" rowspan="1" |6q25.3 | |||
| colspan="1" rowspan="1" |0·92 | |||
| colspan="1" rowspan="1" |[[Signal transduction]] | |||
|- | |||
| colspan="1" rowspan="1" |IKZF1 | |||
| colspan="1" rowspan="1" |7p12.2 | |||
| colspan="1" rowspan="1" |0·89 | |||
| colspan="1" rowspan="1" |Immune-cell regulation | |||
|- | |||
| colspan="1" rowspan="1" |GLIS3 | |||
| colspan="1" rowspan="1" |9p24.2 | |||
| colspan="1" rowspan="1" |1·12 | |||
1·12 | |||
0·90 | |||
| colspan="1" rowspan="1" |Regulation of [[Transcription (genetics)|transcription]] | |||
|- | |||
| colspan="1" rowspan="1" |IL2RA | |||
| colspan="1" rowspan="1" |10p15.1 | |||
| colspan="1" rowspan="1" |1·20 | |||
0·73 | |||
0·52 | |||
0·62 | |||
0·82 | |||
| colspan="1" rowspan="1" |Alternative [[Messenger RNA|mRNA]] splicing [[T helper cell|Th1]] or [[T helper cell|Th2 cell]] [[differentiation]] | |||
|- | |||
| colspan="1" rowspan="1" |PRKCQ | |||
| colspan="1" rowspan="1" |10p15.1 | |||
| colspan="1" rowspan="1" |0·69 | |||
| colspan="1" rowspan="1" |Apoptotic process, inflammatory response, [[innate immune response]], and [[T cell]]-receptor signalling pathway | |||
|- | |||
| colspan="1" rowspan="1" |NRP1 | |||
| colspan="1" rowspan="1" |10p11.22 | |||
| colspan="1" rowspan="1" |1·11 | |||
| colspan="1" rowspan="1" |[[Signal transduction]] | |||
|- | |||
| colspan="1" rowspan="1" |INS | |||
| colspan="1" rowspan="1" |11p15.5 | |||
| colspan="1" rowspan="1" |0·42 | |||
0·63 | |||
0·63 | |||
| colspan="1" rowspan="1" |[[Insulin]] signalling pathway | |||
|- | |||
| colspan="1" rowspan="1" |BAD | |||
| colspan="1" rowspan="1" |11q13.1 | |||
| colspan="1" rowspan="1" |0·92 | |||
| colspan="1" rowspan="1" |[[Apoptosis]] | |||
|- | |||
| colspan="1" rowspan="1" |CD69 | |||
| colspan="1" rowspan="1" |12p13.31 | |||
| colspan="1" rowspan="1" |0·87 | |||
1·10 | |||
| colspan="1" rowspan="1" |[[Signal transduction]] | |||
|- | |||
| colspan="1" rowspan="1" |ITGB7 | |||
| colspan="1" rowspan="1" |12q13.13 | |||
| colspan="1" rowspan="1" |1·19 | |||
| colspan="1" rowspan="1" |Response to [[virus]] and regulation of immune response | |||
|- | |||
| colspan="1" rowspan="1" |ERBB3 | |||
| colspan="1" rowspan="1" |12q13.2 | |||
| colspan="1" rowspan="1" |1·25 | |||
| colspan="1" rowspan="1" |Regulation of [[Transcription (genetics)|transcription]], [[Innate immune system|innate immune response]], and [[lipid metabolism]] | |||
|- | |||
| colspan="1" rowspan="1" |CYP27B1 | |||
| colspan="1" rowspan="1" |12q14.1 | |||
| colspan="1" rowspan="1" |0·82 | |||
| colspan="1" rowspan="1" |[[lipid metabolism|Metabolism of lipids]], [[lipoprotein|lipoproteins]], steroid [[hormone|hormones]], and [[vitamin D]] | |||
|- | |||
| colspan="1" rowspan="1" |SH2B3 | |||
| colspan="1" rowspan="1" |12q24.12 | |||
| colspan="1" rowspan="1" |1·24 | |||
0·76 | |||
0·76 | |||
| colspan="1" rowspan="1" |[[Signal transduction]] | |||
|- | |||
| colspan="1" rowspan="1" |GPR183 | |||
| colspan="1" rowspan="1" |13q32.3 | |||
| colspan="1" rowspan="1" |1·12 | |||
| colspan="1" rowspan="1" |[[Humoral immunity|Humoral immune response]] | |||
|- | |||
| colspan="1" rowspan="1" |DLK1 | |||
| colspan="1" rowspan="1" |14q32.2 | |||
| colspan="1" rowspan="1" |0·88 | |||
0·90 | |||
| colspan="1" rowspan="1" |Regulation of [[gene expression]] | |||
|- | |||
| colspan="1" rowspan="1" |RASGRP1 | |||
| colspan="1" rowspan="1" |15q14 | |||
| colspan="1" rowspan="1" |0·85 | |||
1·15 | |||
| colspan="1" rowspan="1" |Inflammatory response to [[antigen|antigenic]] stimulus and [[cytokine]] production | |||
|- | |||
| colspan="1" rowspan="1" |CTSH | |||
| colspan="1" rowspan="1" |15q25.1 | |||
| colspan="1" rowspan="1" |0·81 | |||
0·78 | |||
0·90 | |||
| colspan="1" rowspan="1" |Immune response-regulating signalling pathway [[T cell]]-mediated [[cytotoxicity]] adaptive immune response | |||
|- | |||
| colspan="1" rowspan="1" |CLEC16A | |||
| colspan="1" rowspan="1" |16p13.13 | |||
| colspan="1" rowspan="1" |0·83 | |||
0·82 | |||
1·14 | |||
| colspan="1" rowspan="1" |Unknown | |||
|- | |||
| colspan="1" rowspan="1" |IL27 | |||
| colspan="1" rowspan="1" |16p11.2 | |||
| colspan="1" rowspan="1" |1·19 | |||
0·90 | |||
1·24 | |||
| colspan="1" rowspan="1" |Inflammatory response and regulation of defence response to [[virus]] | |||
|- | |||
| colspan="1" rowspan="1" |ORMDL3 | |||
| colspan="1" rowspan="1" |17q12 | |||
| colspan="1" rowspan="1" |0·90 | |||
| colspan="1" rowspan="1" |[[Protein]] binding | |||
|- | |||
| colspan="1" rowspan="1" |PTPN2 | |||
| colspan="1" rowspan="1" |18p11.21 | |||
| colspan="1" rowspan="1" |1·20 | |||
| colspan="1" rowspan="1" |[[Cytokine]] signalling and [[B cell]] and [[T cell]] [[differentiation]] | |||
|- | |||
| colspan="1" rowspan="1" |CD226 | |||
| colspan="1" rowspan="1" |18q22.2 | |||
| colspan="1" rowspan="1" |1·13 | |||
| colspan="1" rowspan="1" |Immunoregulation and [[adaptive immune system]] | |||
|- | |||
| colspan="1" rowspan="1" |TYK2 | |||
| colspan="1" rowspan="1" |19p13.2 | |||
| colspan="1" rowspan="1" |0·82 | |||
0·87 | |||
0·67 | |||
| colspan="1" rowspan="1" |[[Cytokine]]-mediated signalling pathway, intracellular [[signal transduction]], and type I [[interferon]] signalling pathway | |||
|- | |||
| colspan="1" rowspan="1" |FUT2 | |||
| colspan="1" rowspan="1" |19q13.33 | |||
| colspan="1" rowspan="1" |0·87 | |||
0·75 | |||
0·87 | |||
| colspan="1" rowspan="1" |Metabolic pathways | |||
|- | |||
| colspan="1" rowspan="1" |UBASH3A | |||
| colspan="1" rowspan="1" |21q22.3 | |||
| colspan="1" rowspan="1" |1·16 | |||
| colspan="1" rowspan="1" |Regulation of [[cytokine]] production | |||
Regulation of [[T cel]]l receptor signalling pathway | |||
|- | |||
| colspan="1" rowspan="1" |C1QTNF6 | |||
| colspan="1" rowspan="1" |22q12.3 | |||
| colspan="1" rowspan="1" |1·11 | |||
| colspan="1" rowspan="1" |[[B cell]] receptor signalling pathway, [[chemokine]] signalling pathway, and [[natural killer cell]]-mediated [[cytotoxicity]] | |||
|} | |||
</SMALL></SMALL> | |||
Some | === Environment === | ||
*Environmental factors were found to influence [[Diabetes mellitus type 1]] through various pathways. Some were found to confer protection against [[Diabetes mellitus type 1]], while others were associated with the progression and promotion of [[Diabetes mellitus type 1]], including:<ref>Volume 387, Issue 10035, 4–10 June 2016, Pages 2340–2348 | |||
Series | |||
Environmental risk factors for type 1 diabetes | |||
Prof Marian Rewers, MD<sup>a</sup>, | |||
Prof Johnny Ludvigsson, MD | |||
</ref><ref name="pmid27545597">{{cite journal| author=Butalia S, Kaplan GG, Khokhar B, Rabi DM| title=Environmental Risk Factors and Type 1 Diabetes: Past, Present, and Future. | journal=Can J Diabetes | year= 2016 | volume= 40 | issue= 6 | pages= 586-593 | pmid=27545597 | doi=10.1016/j.jcjd.2016.05.002 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27545597 }}</ref> | |||
{| class="wikitable" | |||
! | |||
!Triggers | |||
!Protective factors | |||
|- | |||
|[[Obstetrics|Prenatal]] triggers | |||
| | |||
[[ | * [[Congenital rubella|Congenital rubell]]<nowiki/>[[Congenital rubella|a]] | ||
* Maternal [[Enterovirus|Enteroviral]] [[infection]] | |||
* [[Caesarean section|Cesarean section]] | |||
* Higher [[birth weight]] | |||
* Older maternal age | |||
* Low maternal intake of vegetables | |||
| | |||
* Higher maternal [[vitamin D]] intake or [[vitamin D]] concentrations in late [[pregnancy]] | |||
|- | |||
|[[Postnatal]] triggers | |||
| | |||
* [[Enterovirus|Enteroviral infection]] | |||
* Frequent respiratory or enteric infections | |||
* Abnormal [[microbiome]] | |||
* Early exposure to cereals, root vegetables, eggs and cow's milk | |||
* Infant [[weight gain]] | |||
* Serious life events | |||
| | |||
* Probiotic in first month | |||
* Higher [[Omega-3 fatty acid|omega-3 fatty acids]] | |||
* introduction of solid foods while [[breastfeeding]] and after age 4 months | |||
|- | |||
|Promoters of progression | |||
| | |||
* Persistent or recurrent [[Enterovirus|Enteroviral infections]] | |||
* [[Overweight]] or increased height velocity | |||
* High glycemic load, [[fructose]] intake | |||
* Dietary [[nitrates]] or [[Nitrosamine|nitrosamines]] | |||
* [[Puberty]] | |||
* [[Steroid]] [[treatment]] | |||
* [[Insulin resistance]] | |||
* [[Stress (medicine)|Psychological stress]] | |||
| | |||
|} | |||
=== Immunological === | |||
*Several studies have found that abnormalities in the [[Humoral immunity|humoral]] and [[Cell-mediated immunity|cellular arm]] of the [[immune system]], were identified to be associated with [[Diabetes mellitus type 1]], these include:<ref name="pmid26271890">{{cite journal| author=Jaberi-Douraki M, Pietropaolo M, Khadra A| title=Continuum model of T-cell avidity: Understanding autoreactive and regulatory T-cell responses in type 1 diabetes. | journal=J Theor Biol | year= 2015 | volume= 383 | issue= | pages= 93-105 | pmid=26271890 | doi=10.1016/j.jtbi.2015.07.032 | pmc=4567915 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26271890 }}</ref><ref name="pmid24105410">{{cite journal| author=Rydén A, Ludvigsson J, Fredrikson M, Faresjö M| title=General immune dampening is associated with disturbed metabolism at diagnosis of type 1 diabetes. | journal=Pediatr Res | year= 2014 | volume= 75 | issue= 1-1 | pages= 45-50 | pmid=24105410 | doi=10.1038/pr.2013.167 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24105410 }}</ref><ref>Type 1 Diabetes mellitus "Dennis Kasper, Anthony Fauci, Stephen Hauser, Dan Longo, J. Larry Jameson, Joseph Loscalzo"Harrison's Principles of Internal Medicine, 19e Accessed on December 27th,2016</ref><ref name="PaschouPapadopoulou-Marketou20182">{{cite journal|last1=Paschou|first1=Stavroula A|last2=Papadopoulou-Marketou|first2=Nektaria|last3=Chrousos|first3=George P|last4=Kanaka-Gantenbein|first4=Christina|title=On type 1 diabetes mellitus pathogenesis|journal=Endocrine Connections|volume=7|issue=1|year=2018|pages=R38–R46|issn=2049-3614|doi=10.1530/EC-17-0347}}</ref><ref name="pmid9568688">{{cite journal| author=Ellis TM, Schatz DA, Ottendorfer EW, Lan MS, Wasserfall C, Salisbury PJ | display-authors=etal| title=The relationship between humoral and cellular immunity to IA-2 in IDDM. | journal=Diabetes | year= 1998 | volume= 47 | issue= 4 | pages= 566-9 | pmid=9568688 | doi=10.2337/diabetes.47.4.566 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9568688 }}</ref><ref name="pmid23890997">{{cite journal| author=Atkinson MA, Eisenbarth GS, Michels AW| title=Type 1 diabetes. | journal=Lancet | year= 2014 | volume= 383 | issue= 9911 | pages= 69-82 | pmid=23890997 | doi=10.1016/S0140-6736(13)60591-7 | pmc=4380133 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23890997 }} </ref> | |||
** Deficiency in immune regulation, such as effector [[T cell|T cells]] (Teff) resistance to Regulatory [[T cell|T cells]] (Treg) or Regulatory [[T cell|T cells]] (Treg) abnormalities | |||
** [[Islets of Langerhans|Islet cell]] [[Autoantibody|autoantibodies]] | |||
** Defective cellular trafficking and adhesion | |||
** Activated [[lymphocytes]] in the [[Islets of Langerhans|islets]], peripancreatic [[lymph node|lymph nodes]], and systemic circulation | |||
** Chronic activation of [[Antigen-presenting cell|Antigen-presenting cells]] | |||
** [[T lymphocytes]] that proliferate when stimulated with [[Islets of Langerhans|islet]] [[protein|proteins]] | |||
** Release of [[cytokines]] within the insulitis | |||
** An [[enzyme]] named [[Glutamate decarboxylase|glutamic acid decarboxylase]] ([[Glutamate decarboxylase|GAD65]]) found in [[Beta cell|β cells]] has similar [[amino acid sequence]] with the [[Coxsackie virus|Coxsackie]] B4 P2-C [[protein]], which augments the response of [[humoral immunity]]. | |||
** [[Autoantibody|Autoantibodies]] against IA-2 and [[zinc]] transporter (ZnT8) have been positive in 60% and 60-80% of [[Diabetes mellitus type 1]] at the time of [[Diagnosis|diagnose]], respectively. | |||
=== Associated conditions === | |||
* Conditions associated with [[diabetes mellitus type 1]] include:<ref name=":0" /><ref name="pmid22516771">{{cite journal| author=Witek PR, Witek J, Pańkowska E| title=[Type 1 diabetes-associated autoimmune diseases: screening, diagnostic principles and management]. | journal=Med Wieku Rozwoj | year= 2012 | volume= 16 | issue= 1 | pages= 23-34 | pmid=22516771 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22516771 }}</ref> | |||
** [[Autoimmunity|Autoimmune]] [[thyroid disease]] (ATD) | |||
** [[Celiac disease|Celiac disease]] ([[Celiac disease|CD]]) | |||
** [[Gastritis|Autoimmune gastritis]] (AIG) | |||
** [[Pernicious anemia]] ([[Pernicious anemia|PA]]) | |||
** [[Vitiligo]] | |||
** [[Addison's disease]] | |||
* The following table is a summary of some associated [[Autoimmunity|autoimmune]] conditions and their prevalence among [[type 1 diabetes mellitus|type 1 diabetic]] patients and normal population.<ref name="NederstigtUitbeijerse2019">{{cite journal|last1=Nederstigt|first1=C|last2=Uitbeijerse|first2=B S|last3=Janssen|first3=L G M|last4=Corssmit|first4=E P M|last5=de Koning|first5=E J P|last6=Dekkers|first6=O M|title=Associated auto-immune disease in type 1 diabetes patients: a systematic review and meta-analysis|journal=European Journal of Endocrinology|volume=180|issue=2|year=2019|pages=135–144|issn=0804-4643|doi=10.1530/EJE-18-0515}}</ref> | |||
{| class="wikitable" | |||
|+ | |||
!Associated [[Autoimmunity|autoimmune]] conditions | |||
![[Prevalence]] in patients with [[diabetes mellitus type 1]] (%) | |||
!95% [[Confidence interval|CI]] | |||
![[Prevalence]] in the general population (%) | |||
|- | |||
|[[Hypothyroidism]] | |||
|9.8 | |||
|7.5–12.3 | |||
|2–4.6 | |||
|- | |||
|Positive [[Thyroid peroxidase|TPO]] and or [[Thyroglobulin|TG]] [[antibody|antibodies]] | |||
|18.9 | |||
|17.2–20.6 | |||
|Unknown | |||
|- | |||
|Positive [[Thyroid peroxidase|TPO]] [[antibody|antibodies]] | |||
|18.3 | |||
|15.8–21.0 | |||
|11.3–12.8 | |||
|- | |||
|Positive [[Thyroglobulin|TG]] [[antibody|antibodies]] | |||
|12.3 | |||
|10.0–14.9 | |||
|10.4 | |||
|- | |||
|[[Hyperthyroidism]] | |||
|1.3 | |||
|0.9–1.8 | |||
|1.0–4.0 | |||
|- | |||
|Positive [[Thyrotropin receptor|TSH receptor]] [[antibody|antibodies]] and or Thyroid stimulating immunoglobulin | |||
|9.5 | |||
|1.4–22.7 | |||
|Unknown | |||
|- | |||
|[[Celiac disease]] | |||
|4.7 | |||
|4.0–5.5 | |||
|0.5–1.0 | |||
|- | |||
|Presence of any [[gluten]] related [[antibody|antibodies]] | |||
|10.2 | |||
|8.4–12.7 | |||
|Unknown | |||
|- | |||
|Positive [[tissue transglutaminase]] [[antibody|antibodies]] ([[Immunoglobulin A|IgA]]) | |||
|9.8 | |||
|8.2–11.6 | |||
|1.5 | |||
|- | |||
|Positive [[tissue transglutaminase]] [[antibody|antibodies]] ([[Immunoglobulin A|IgA]]/[[Immunoglobulin G|IgG]]) | |||
|9.8 | |||
|8.4–11.3 | |||
|2.1 | |||
|- | |||
|Positive anti-endomysial [[antibody|antibodies]] ([[Immunoglobulin A|IgA]]) | |||
|5.3 | |||
|4.3–6.4 | |||
|0.8 | |||
|- | |||
|Positive antigliadin [[antibody|antibodies]] ([[Immunoglobulin A|IgA]]) | |||
|9.7 | |||
|5.1–15.5 | |||
|1.6 | |||
|- | |||
|Positive antigliadin [[antibody|antibodies]] ([[Immunoglobulin G|IgG]]) | |||
|12.7 | |||
|6.1–21.0 | |||
|7.1 | |||
|- | |||
|[[Pernicious anemia]] | |||
|4.3 | |||
|1.6–8.2 | |||
|0.2 | |||
|- | |||
|Positive anti-[[parietal cell]] [[antibody|antibodies]] | |||
|9.3 | |||
|5.4–14.1 | |||
|3–10 | |||
|- | |||
|[[Vitiligo]] | |||
|2.4 | |||
|1.2–3.9 | |||
|0.4 | |||
|- | |||
|[[Adrenal insufficiency|Adrenal gland insufficiency]] | |||
|0.2 | |||
|0.0–0.4 | |||
|0.012 | |||
|- | |||
|Positive anti-adrenal [[antibody|antibodies]] (AAA/21-OHab) | |||
|1.4 | |||
|0.8–2.2 | |||
|Unknown | |||
|} | |||
==Gross Pathology== | |||
*On [[gross pathology]], [[pancreas]] may demonstrated the following changes:<ref name="pmid23890997">{{cite journal| author=Atkinson MA, Eisenbarth GS, Michels AW| title=Type 1 diabetes. | journal=Lancet | year= 2014 | volume= 383 | issue= 9911 | pages= 69-82 | pmid=23890997 | doi=10.1016/S0140-6736(13)60591-7 | pmc=4380133 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23890997 }} </ref> | |||
** Decreased overall weight and size | |||
** Dorsal region [[atrophy]] | |||
** Possible [[Hypertrophy (medical)|hypertrophy]] (related to hydrophic changes) | |||
==Microscopic Pathology== | |||
*On microscopic [[Histopathology|histopathological]] [[analysis]], the following changes can be detected in [[Islets of Langerhans|islet cells]]:<ref name="pmid23890997">{{cite journal| author=Atkinson MA, Eisenbarth GS, Michels AW| title=Type 1 diabetes. | journal=Lancet | year= 2014 | volume= 383 | issue= 9911 | pages= 69-82 | pmid=23890997 | doi=10.1016/S0140-6736(13)60591-7 | pmc=4380133 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23890997 }} </ref> | |||
** Insulitis | |||
** [[Beta cell]] loss due to [[necrosis]] or [[apoptosis]] | |||
** [[Major histocompatibility complex]] class one hyperexpression | |||
** Reduction in [[insulin]] in remnant [[Beta cell|beta cells]] | |||
** [[Interferon type I|Interferon alpha]] [[Gene expression|expression]] in [[Beta cell|beta cells]] | |||
** [[CD3 (immunology)|CD3]]-positive cells in [[Islets of Langerhans|Islet cell]] | |||
==References== | ==References== | ||
{{reflist|2}}{{WH}} {{WS}} {{WH}} {{WS}} | |||
__NOTOC__ | |||
{{reflist|2}} | {{reflist|2}} | ||
{{WH}} | {{WH}} | ||
{{WS}} | {{WS}} | ||
{{WH}} | {{WH}} | ||
{{WS}} | {{WS}} | ||
Latest revision as of 10:31, 31 August 2020
|
Diabetes mellitus type 1 Microchapters |
|
Differentiating Diabetes mellitus type 1 from other Diseases |
|
Diagnosis |
|
Treatment |
|
Cardiovascular Disease and Risk Management |
|
Case Studies |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Priyamvada Singh, M.B.B.S. [2]; Cafer Zorkun, M.D., Ph.D. [3]Vishal Devarkonda, M.B.B.S[4] Anahita Deylamsalehi, M.D.[5]
Overview
Type 1 diabetes is a disorder characterized by abnormally high blood sugar levels. Type 1 diabetes is the result of interactions of genetic, environmental, and immunologic factors that ultimately lead to the destruction of the pancreatic beta cells and insulin deficiency. Currently, 58 genomic regions are known to be associated with type 1 DM. There are environmental factors that can play a protective role in Type 1 diabetes like higher maternal vitamin D, probiotic and omega-3 fatty acids intake during prenatal period. Conversely, environmental factors such as caesarean section, congenital rubella, maternal enteroviral infection and abnormal microbiome are among environmental factors that are able to trigger Type 1 DM. Furthermore, some immunological components are responsible for type 1 DM pathogenesis.
Pathophysiology
- Type 1 diabetes is a disorder characterized by abnormally high blood sugar levels.
- In this form of diabetes, specialized cells in the pancreas called beta cells stop producing insulin. Insulin controls how much glucose (a type of sugar) is passed from the blood into cells for conversion to energy. Lack of insulin results in the inability to use glucose for energy or to control the amount of sugar in the blood.
- Possible thymus and bone marrow deficiency includes defective thymic selection, faulty self antigen presentation, thymic VNTR and Aire expression, mobilopathy and defective lymphocyte precursors are some possible proceeding triggers that underlie type 1 DM.[1]
Pathogenesis
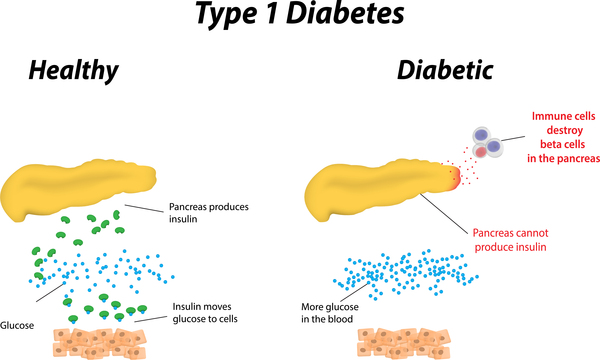
- Type 1 DM is the result of interactions of genetic, environmental, and immunologic factors that ultimately lead to the destruction of the pancreatic beta cells and insulin deficiency.
- Concordance of type 1 DM in identical twins ranges between 40 and 60%, indicating the presence of additional modifying factors.
Genetics
- Genes associated with Diabetes mellitus include the following: [2][3][4][5][6][7]
- Currently, 58 genomic regions are known to be associated with Type 1 DM.
- Major susceptibility gene for type 1 diabetes is located on HLA region of chromosome 6. It accounts for 40-50% of the genetic risk for type 1 diabetes. This region encodes for class II major histocompatibility complex (MHC) molecules. major histocompatibility complex (MHC) molecules play an important role in presenting antigen to T helper cell and initiating immune response.
- Other major susceptibility genes which were associated with Type 1 DM include polymorphisms in the promoter region of the insulin gene, the CTLA-4 gene, interleukin 2 receptor, Insulin-VNTR, AIRE, FoxP3, STAT3, HIP14 and PTPN22 etc.[8][9]
- Presence of certain genes confer protection against the development of the disease. Haplotype DQA1*0102, DQB1*0602 is extremely rare in individuals with type 1 DM (<1%) and appears to provide protection from type 1 diabetes.
- There is a relationship between some human leukocyte antigens (HLA) and type 1 diabetes, such as DQB1, DQA1, and DRB1. There are some supporting data on DR4-linked haplotypes transmission from type 2 diabetes parents to offspring with type 1 diabetes. Patients with latent autoimmune diabetes of adults also have been related to HLA alleles DQB1*0302 and 02. [9]
| Genes important to type 1 diabetes pathogenesis | Region | Odds ratio | Gene funtion |
|---|---|---|---|
| PTPN22 | 1p13.2 | 1·89 | Regulation of innate immune response, T cell activation, and natural killer cell proliferation |
| IL10 | 1q32.1 | 0·86 | Cytokines and inflammatory response |
| AFF3 | 2q11.2 | 1·11 | Regulation of transcription |
| IFIH1 | 2q24.2 | 0·85
0·85 0·59 |
Innate immune system NF-κB activation |
| STAT4 | 2q32.3 | 1·10§ | Cytokine-mediated signalling pathway |
| CTLA4 | 2q33.2 | 0·82
0·84 |
T cell activation |
| CCR5 | 3p21.31 | 0·85 | Th1 cell development and chemokine-mediated signalling pathway |
| IL21, IL2 | 4q27 | 1·13
1·12 1·14 1·15 |
Cytokines and inflammatory response and Th1 cell or Th2 cell differentiation |
| IL7R | 5p13.2 | 1·11 | T cell-mediated cytotoxicity, immunoglobulin production, and antigen binding |
| BACH2 | 6q15 | 1·10
0·88 1·20 |
transcription |
| TNFAIP3 | 6q23.3 | 1·12 | Inflammatory response |
| TAGAP | 6q25.3 | 0·92 | Signal transduction |
| IKZF1 | 7p12.2 | 0·89 | Immune-cell regulation |
| GLIS3 | 9p24.2 | 1·12
1·12 0·90 |
Regulation of transcription |
| IL2RA | 10p15.1 | 1·20
0·73 0·52 0·62 0·82 |
Alternative mRNA splicing Th1 or Th2 cell differentiation |
| PRKCQ | 10p15.1 | 0·69 | Apoptotic process, inflammatory response, innate immune response, and T cell-receptor signalling pathway |
| NRP1 | 10p11.22 | 1·11 | Signal transduction |
| INS | 11p15.5 | 0·42
0·63 0·63 |
Insulin signalling pathway |
| BAD | 11q13.1 | 0·92 | Apoptosis |
| CD69 | 12p13.31 | 0·87
1·10 |
Signal transduction |
| ITGB7 | 12q13.13 | 1·19 | Response to virus and regulation of immune response |
| ERBB3 | 12q13.2 | 1·25 | Regulation of transcription, innate immune response, and lipid metabolism |
| CYP27B1 | 12q14.1 | 0·82 | Metabolism of lipids, lipoproteins, steroid hormones, and vitamin D |
| SH2B3 | 12q24.12 | 1·24
0·76 0·76 |
Signal transduction |
| GPR183 | 13q32.3 | 1·12 | Humoral immune response |
| DLK1 | 14q32.2 | 0·88
0·90 |
Regulation of gene expression |
| RASGRP1 | 15q14 | 0·85
1·15 |
Inflammatory response to antigenic stimulus and cytokine production |
| CTSH | 15q25.1 | 0·81
0·78 0·90 |
Immune response-regulating signalling pathway T cell-mediated cytotoxicity adaptive immune response |
| CLEC16A | 16p13.13 | 0·83
0·82 1·14 |
Unknown |
| IL27 | 16p11.2 | 1·19
0·90 1·24 |
Inflammatory response and regulation of defence response to virus |
| ORMDL3 | 17q12 | 0·90 | Protein binding |
| PTPN2 | 18p11.21 | 1·20 | Cytokine signalling and B cell and T cell differentiation |
| CD226 | 18q22.2 | 1·13 | Immunoregulation and adaptive immune system |
| TYK2 | 19p13.2 | 0·82
0·87 0·67 |
Cytokine-mediated signalling pathway, intracellular signal transduction, and type I interferon signalling pathway |
| FUT2 | 19q13.33 | 0·87
0·75 0·87 |
Metabolic pathways |
| UBASH3A | 21q22.3 | 1·16 | Regulation of cytokine production
Regulation of T cell receptor signalling pathway |
| C1QTNF6 | 22q12.3 | 1·11 | B cell receptor signalling pathway, chemokine signalling pathway, and natural killer cell-mediated cytotoxicity |
Environment
- Environmental factors were found to influence Diabetes mellitus type 1 through various pathways. Some were found to confer protection against Diabetes mellitus type 1, while others were associated with the progression and promotion of Diabetes mellitus type 1, including:[10][11]
| Triggers | Protective factors | |
|---|---|---|
| Prenatal triggers |
|
|
| Postnatal triggers |
|
|
| Promoters of progression |
|
Immunological
- Several studies have found that abnormalities in the humoral and cellular arm of the immune system, were identified to be associated with Diabetes mellitus type 1, these include:[12][13][14][15][16][1]
- Deficiency in immune regulation, such as effector T cells (Teff) resistance to Regulatory T cells (Treg) or Regulatory T cells (Treg) abnormalities
- Islet cell autoantibodies
- Defective cellular trafficking and adhesion
- Activated lymphocytes in the islets, peripancreatic lymph nodes, and systemic circulation
- Chronic activation of Antigen-presenting cells
- T lymphocytes that proliferate when stimulated with islet proteins
- Release of cytokines within the insulitis
- An enzyme named glutamic acid decarboxylase (GAD65) found in β cells has similar amino acid sequence with the Coxsackie B4 P2-C protein, which augments the response of humoral immunity.
- Autoantibodies against IA-2 and zinc transporter (ZnT8) have been positive in 60% and 60-80% of Diabetes mellitus type 1 at the time of diagnose, respectively.
Associated conditions
- Conditions associated with diabetes mellitus type 1 include:[7][17]
- The following table is a summary of some associated autoimmune conditions and their prevalence among type 1 diabetic patients and normal population.[18]
| Associated autoimmune conditions | Prevalence in patients with diabetes mellitus type 1 (%) | 95% CI | Prevalence in the general population (%) |
|---|---|---|---|
| Hypothyroidism | 9.8 | 7.5–12.3 | 2–4.6 |
| Positive TPO and or TG antibodies | 18.9 | 17.2–20.6 | Unknown |
| Positive TPO antibodies | 18.3 | 15.8–21.0 | 11.3–12.8 |
| Positive TG antibodies | 12.3 | 10.0–14.9 | 10.4 |
| Hyperthyroidism | 1.3 | 0.9–1.8 | 1.0–4.0 |
| Positive TSH receptor antibodies and or Thyroid stimulating immunoglobulin | 9.5 | 1.4–22.7 | Unknown |
| Celiac disease | 4.7 | 4.0–5.5 | 0.5–1.0 |
| Presence of any gluten related antibodies | 10.2 | 8.4–12.7 | Unknown |
| Positive tissue transglutaminase antibodies (IgA) | 9.8 | 8.2–11.6 | 1.5 |
| Positive tissue transglutaminase antibodies (IgA/IgG) | 9.8 | 8.4–11.3 | 2.1 |
| Positive anti-endomysial antibodies (IgA) | 5.3 | 4.3–6.4 | 0.8 |
| Positive antigliadin antibodies (IgA) | 9.7 | 5.1–15.5 | 1.6 |
| Positive antigliadin antibodies (IgG) | 12.7 | 6.1–21.0 | 7.1 |
| Pernicious anemia | 4.3 | 1.6–8.2 | 0.2 |
| Positive anti-parietal cell antibodies | 9.3 | 5.4–14.1 | 3–10 |
| Vitiligo | 2.4 | 1.2–3.9 | 0.4 |
| Adrenal gland insufficiency | 0.2 | 0.0–0.4 | 0.012 |
| Positive anti-adrenal antibodies (AAA/21-OHab) | 1.4 | 0.8–2.2 | Unknown |
Gross Pathology
- On gross pathology, pancreas may demonstrated the following changes:[1]
- Decreased overall weight and size
- Dorsal region atrophy
- Possible hypertrophy (related to hydrophic changes)
Microscopic Pathology
- On microscopic histopathological analysis, the following changes can be detected in islet cells:[1]
- Insulitis
- Beta cell loss due to necrosis or apoptosis
- Major histocompatibility complex class one hyperexpression
- Reduction in insulin in remnant beta cells
- Interferon alpha expression in beta cells
- CD3-positive cells in Islet cell
References
- ↑ 1.0 1.1 1.2 1.3 Atkinson MA, Eisenbarth GS, Michels AW (2014). "Type 1 diabetes". Lancet. 383 (9911): 69–82. doi:10.1016/S0140-6736(13)60591-7. PMC 4380133. PMID 23890997.
- ↑ Pociot F, Lernmark Å (2016). "Genetic risk factors for type 1 diabetes". Lancet. 387 (10035): 2331–9. doi:10.1016/S0140-6736(16)30582-7. PMID 27302272.
- ↑ Safari-Alighiarloo N, Taghizadeh M, Tabatabaei SM, Shahsavari S, Namaki S, Khodakarim S; et al. (2016). "Identification of new key genes for type 1 diabetes through construction and analysis of protein-protein interaction networks based on blood and pancreatic islet transcriptomes". J Diabetes. doi:10.1111/1753-0407.12483. PMID 27625010.
- ↑ Brorsson CA, Pociot F, Type 1 Diabetes Genetics Consortium. Shared genetic basis for type 1 diabetes, islet autoantibodies, and autoantibodies associated with other immune-mediated diseases in families with type 1 diabetes. Diabetes Care 2015; 38 (suppl 3): S8–13.
- ↑ Ahlqvist E, van Zuydam NR, Groop LC, McCarthy MI. The genetics of diabetic complications. Nat Rev Nephrol 2015; 11: 277–87.
- ↑ Parkes M, Cortes A, van Heel DA, Brown MA. Genetic insights into common pathways and complex relationships among immune-mediated diseases. Nat Rev Genet 2013; 14: 661–73.
- ↑ 7.0 7.1 Type 1 Diabetes mellitus "Dennis Kasper, Anthony Fauci, Stephen Hauser, Dan Longo, J. Larry Jameson, Joseph Loscalzo"Harrison's Principles of Internal Medicine, 19e Accessed on December 27th,2016
- ↑ Paschou, Stavroula A; Papadopoulou-Marketou, Nektaria; Chrousos, George P; Kanaka-Gantenbein, Christina (2018). "On type 1 diabetes mellitus pathogenesis". Endocrine Connections. 7 (1): R38–R46. doi:10.1530/EC-17-0347. ISSN 2049-3614.
- ↑ 9.0 9.1 Tuomi, T. (2005). "Type 1 and Type 2 Diabetes: What Do They Have in Common?". Diabetes. 54 (Supplement 2): S40–S45. doi:10.2337/diabetes.54.suppl_2.S40. ISSN 0012-1797.
- ↑ Volume 387, Issue 10035, 4–10 June 2016, Pages 2340–2348 Series Environmental risk factors for type 1 diabetes Prof Marian Rewers, MDa, Prof Johnny Ludvigsson, MD
- ↑ Butalia S, Kaplan GG, Khokhar B, Rabi DM (2016). "Environmental Risk Factors and Type 1 Diabetes: Past, Present, and Future". Can J Diabetes. 40 (6): 586–593. doi:10.1016/j.jcjd.2016.05.002. PMID 27545597.
- ↑ Jaberi-Douraki M, Pietropaolo M, Khadra A (2015). "Continuum model of T-cell avidity: Understanding autoreactive and regulatory T-cell responses in type 1 diabetes". J Theor Biol. 383: 93–105. doi:10.1016/j.jtbi.2015.07.032. PMC 4567915. PMID 26271890.
- ↑ Rydén A, Ludvigsson J, Fredrikson M, Faresjö M (2014). "General immune dampening is associated with disturbed metabolism at diagnosis of type 1 diabetes". Pediatr Res. 75 (1–1): 45–50. doi:10.1038/pr.2013.167. PMID 24105410.
- ↑ Type 1 Diabetes mellitus "Dennis Kasper, Anthony Fauci, Stephen Hauser, Dan Longo, J. Larry Jameson, Joseph Loscalzo"Harrison's Principles of Internal Medicine, 19e Accessed on December 27th,2016
- ↑ Paschou, Stavroula A; Papadopoulou-Marketou, Nektaria; Chrousos, George P; Kanaka-Gantenbein, Christina (2018). "On type 1 diabetes mellitus pathogenesis". Endocrine Connections. 7 (1): R38–R46. doi:10.1530/EC-17-0347. ISSN 2049-3614.
- ↑ Ellis TM, Schatz DA, Ottendorfer EW, Lan MS, Wasserfall C, Salisbury PJ; et al. (1998). "The relationship between humoral and cellular immunity to IA-2 in IDDM". Diabetes. 47 (4): 566–9. doi:10.2337/diabetes.47.4.566. PMID 9568688.
- ↑ Witek PR, Witek J, Pańkowska E (2012). "[Type 1 diabetes-associated autoimmune diseases: screening, diagnostic principles and management]". Med Wieku Rozwoj. 16 (1): 23–34. PMID 22516771.
- ↑ Nederstigt, C; Uitbeijerse, B S; Janssen, L G M; Corssmit, E P M; de Koning, E J P; Dekkers, O M (2019). "Associated auto-immune disease in type 1 diabetes patients: a systematic review and meta-analysis". European Journal of Endocrinology. 180 (2): 135–144. doi:10.1530/EJE-18-0515. ISSN 0804-4643.
Template:WH Template:WS Template:WH Template:WS