Water (molecule)
Water (H2O, HOH) is the most abundant molecule on Earth's surface, composing of about 70% of the Earth's surface as liquid and solid state in addition to being found in the atmosphere as a vapor. It is in dynamic equilibrium between the liquid and vapor states at standard temperature and pressure. At room temperature, it is a nearly colorless, tasteless, and odorless liquid, with a hint of blue. Many substances dissolve in water and it is commonly referred to as the universal solvent. Because of this, water in nature and in use is rarely clean, and may have some properties different from those in the laboratory. However, there are many compounds that are essentially, if not completely, insoluble in water. Water is the only common, pure substance found naturally in all three common states of matter—for other substances, see Chemical properties.
Forms of water
- See the Water#Overview of types of water
Water can take many forms. The solid state of water is commonly known as ice (while many other forms exist; see amorphous solid water); the gaseous state is known as water vapor (or steam, though this is actually incorrect, since steam is just condensing liquid water droplets), and the common liquid phase is generally taken as simply water. Above a certain critical temperature and pressure (647 K and 22.064 MPa), water molecules assume a supercritical condition, in which liquid-like clusters float within a vapor-like phase.
Heavy water is water in which the hydrogen is replaced by its heavier isotope, deuterium. It is chemically almost identical to normal water. Heavy water is used in the nuclear industry to slow down neutrons.
Physics and chemistry of water
Water is the chemical substance with chemical formula H2O: one molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom. Water is a tasteless, odorless liquid at ambient temperature and pressure, and appears colorless in small quantities, although it has its own intrinsic very light blue hue. Ice also appears colorless, and water vapor is essentially invisible as a gas.[1] Water is primarily a liquid under standard conditions, which is not predicted from its relationship to other analogous hydrides of the oxygen family in the periodic table, which are gases such as hydrogen sulfide. Also the elements surrounding oxygen in the periodic table, nitrogen, fluorine, phosphorus, sulfur and chlorine, all combine with hydrogen to produce gases under standard conditions. The reason that oxygen hydride (water) forms a liquid is that it is more electronegative than all of these elements (other than fluorine). Oxygen attracts electrons much more strongly than hydrogen, resulting in a net positive charge on the hydrogen atoms, and a net negative charge on the oxygen atom. The presence of a charge on each of these atoms gives each water molecule a net dipole moment. Electrical attraction between water molecules due to this dipole pulls individual molecules closer together, making it more difficult to separate the molecules and therefore raising the boiling point. This attraction is known as hydrogen bonding. Water can be described as a polar liquid that dissociates disproportionately into the hydronium ion (H3O+(aq)) and an associated hydroxide ion (OH−(aq)). Water is in dynamic equilibrium between the liquid, gas and solid states at standard temperature and pressure, and is the only pure substance found naturally on Earth to be so.
Water, ice and vapor
Heat capacity and heat of vaporization
Water has the second highest specific heat capacity of any known chemical compound, after ammonia, as well as a high heat of vaporization (40.65 kJ mol−1), both of which are a result of the extensive hydrogen bonding between its molecules. These two unusual properties allow water to moderate Earth's climate by buffering large fluctuations in temperature.
Density of water and ice
The solid form of most substances is more dense than the liquid phase; thus, a block of pure solid substance will sink in a tub of pure liquid substance. But, by contrast, a block of common ice will float in a tub of water because solid water is less dense than liquid water. This is an extremely important characteristic property of water. At room temperature, liquid water becomes denser with lowering temperature, just like other substances. But at 4 °C, just above freezing, water reaches its maximum density, and as water cools further toward its freezing point, the liquid water, under standard conditions, expands to become less dense. The physical reason for this is related to the crystal structure of ordinary ice, known as hexagonal ice Ih. Water, lead, uranium, neon and silicon are some of the few materials which expand when they freeze; most other materials contract. It should be noted however, that not all forms of ice are less dense than liquid water. For example HDA and VHDA are both more dense than liquid phase pure water. Thus, the reason that the common form of ice is less dense than water is a bit non-intuitive and relies heavily on the unusual properties inherent to the hydrogen bond.
Generally, water expands when it freezes because of its molecular structure, in tandem with the unusual elasticity of the hydrogen bond and the particular lowest energy hexagonal crystal conformation that it adopts under standard conditions. That is, when water cools, it tries to stack in a crystalline lattice configuration that stretches the rotational and vibrational components of the bond, so that the effect is that each molecule of water is pushed further from each of its neighboring molecules. This effectively reduces the density ρ of water when ice is formed under standard conditions.
The importance of this property cannot be overemphasized for its role on the ecosystem of Earth. For example, if water were more dense when frozen, lakes and oceans in a polar environment would eventually freeze solid (from top to bottom). This would happen because frozen ice would settle on the lake and riverbeds, and the necessary warming phenomenon (see below) could not occur in summer, as the warm surface layer would be less dense than the solid frozen layer below. It is a significant feature of nature that this does not occur naturally in the environment.
Nevertheless, the unusual expansion of freezing water (in ordinary natural settings in relevant biological systems), due to the hydrogen bond, from 4 °C above freezing to the freezing point offers an important advantage for freshwater life in winter. Water chilled at the surface increases in density and sinks, forming convection currents that cool the whole water body, but when the temperature of the lake water reaches 4 °C, water on the surface decreases in density as it chills further and remains as a surface layer which eventually freezes and forms ice. Since downward convection of colder water is blocked by the density change, any large body of fresh water frozen in winter will have the coldest water near the surface, away from the riverbed or lakebed. This accounts for various little known phenomena of ice characteristics as they relate to ice in lakes and "ice falling out of lakes" as described by early 20th century scientist Horatio D. Craft.
The following table gives the density of water in grams per cubic centimeter at various temperatures in degrees Celsius:[2]
| Temp (°C) | Density (g/cm³) |
|---|---|
| 30 | 0.9956502 |
| 25 | 0.9970479 |
| 22 | 0.9977735 |
| 20 | 0.9982071 |
| 15 | 0.9991026 |
| 10 | 0.9997026 |
| 4 | 0.9999720 |
| 0 | 0.9998395 |
| −10 | 0.998117 |
| −20 | 0.993547 |
| −30 | 0.983854 |
The values below 0 °C refer to supercooled water.
- Freezing point
A simple but environmentally important and unusual property of water is that its usual solid form, ice, floats on its liquid form. This solid state is not as dense as liquid water because of the geometry of the hydrogen bonds which are formed only at lower temperatures. For almost all other substances the solid form has a greater density than the liquid form. Fresh water at standard atmospheric pressure is most dense at 3.98 °C, and will sink by convection as it cools to that temperature, and if it becomes colder it will rise instead. This reversal will cause deep water to remain warmer than shallower freezing water, so that ice in a body of water will form first at the surface and progress downward, while the majority of the water underneath will hold a constant 4 °C. This effectively insulates a lake floor from the cold. The water will freeze at 0 °C (32 °F, 273 K), however, it can be supercooled in a fluid state down to its crystal homogeneous nucleation at almost 231 K (−42 °C)[3]. Ice also has a number of more exotic phases not commonly seen (go to the full article on Ice).
Density of saltwater and ice
The density of water is dependent on the dissolved salt content as well as the temperature of the water. Ice still floats in the oceans, otherwise they would freeze from the bottom up. However, the salt content of oceans lowers the freezing point by about 2 °C and lowers the temperature of the density maximum of water to the freezing point. That is why, in ocean water, the downward convection of colder water is not blocked by an expansion of water as it becomes colder near the freezing point. The oceans' cold water near the freezing point continues to sink. For this reason, any creature attempting to survive at the bottom of such cold water as the Arctic Ocean generally lives in water that is 4 °C colder than the temperature at the bottom of frozen-over fresh water lakes and rivers in the winter.
As the surface of salt water begins to freeze (at −1.9 °C for normal salinity seawater, 3.5%) the ice that forms is essentially salt free with a density approximately equal to that of freshwater ice. This ice floats on the surface and the salt that is "frozen out" adds to the salinity and density of the seawater just below it, in a process known as brine rejection. This more dense saltwater sinks by convection and the replacing seawater is subject to the same process. This provides essentially freshwater ice at −1.9 °C on the surface. The increased density of the seawater beneath the forming ice causes it to sink towards the bottom.
Miscibility and condensation
Water is miscible with many liquids, for example ethanol in all proportions, forming a single homogeneous liquid. On the other hand water and most oils are immiscible usually forming layers according to increasing density from the top.
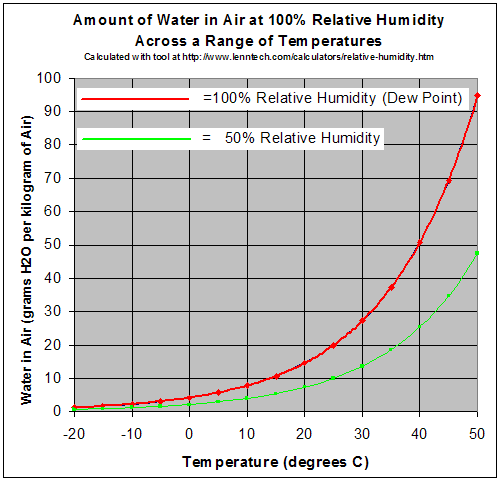
As a gas, water vapor is completely miscible with air. On the other hand the maximum water vapor pressure that is thermodynamically stable with the liquid (or solid) at a given temperature is relatively low compared with total atmospheric pressure. For example, if the vapor partial pressure[4] is 2% of atmospheric pressure and the air is cooled from 25 °C, starting at about 22 °C water will start to condense, defining the dew point, and creating fog or dew. The reverse process accounts for the fog burning off in the morning. If one raises the humidity at room temperature, say by running a hot shower or a bath, and the temperature stays about the same, the vapor soon reaches the pressure for phase change, and condenses out as steam. A gas in this context is referred to as saturated or 100% relative humidity, when the vapor pressure of water in the air is at the equilibrium with vapor pressure due to (liquid) water; water (or ice, if cool enough) will fail to lose mass through evaporation when exposed to saturated air. Because the amount of water vapor in air is small, relative humidity, the ratio of the partial pressure due to the water vapor to the saturated partial vapor pressure, is much more useful. Water vapor pressure above 100% relative humidity is called super-saturated and can occur if air is rapidly cooled, say by rising suddenly in an updraft.[5]
Vapor Pressures of Water
| Temperature (°C) | Pressure (torr) |
|---|---|
| 0 | 4.58 |
| 5 | 6.54 |
| 10 | 9.21 |
| 12 | 10.52 |
| 14 | 11.99 |
| 16 | 13.63 |
| 17 | 14.53 |
| 18 | 15.48 |
| 19 | 16.48 |
| 20 | 17.54 |
| 21 | 18.65 |
| 22 | 19.83 |
| 23 | 21.07 |
| 24 | 22.38 |
| 25 | 23.76 |
Compressibility
The compressibility of water is a function of pressure and temperature. At 0 °C in the limit of zero pressure the compressibility is 5.1×10-5 bar−1.[7] In the zero pressure limit the compressibility reaches a minimum of 4.4×10-5 bar−1 around 45 °C before increasing again with increasing temperature. As the pressure is increased the compressibility decreases, being 3.9×10-5 bar−1 at 0 °C and 1000 bar. The bulk modulus of water is 2.2×109 Pa.[8] The low compressibility of non-gases, and of water in particular, leads to them often being assumed as incompressible. The low compressibility of water means that even in the deep oceans at 4000 m depth, where pressures are 4×107 Pa, there is only a 1.8% decrease in volume.[8]
Triple point
| Phases in stable equilibrium | Pressure | Temperature |
|---|---|---|
| liquid water, ice I, and water vapour | 611.73 Pa | 273.16 K |
| liquid water, ice Ih, and ice III | 209.9 MPa | 251 K (-22 °C) |
| liquid water, ice Ih, and gaseous water | 612 Pa | 0.01 °C |
| liquid water, ice III, and ice V | 350.1 MPa | -17.0 °C |
| liquid water, ice V, and ice VI | 632.4 MPa | 0.16 °C |
| ice Ih, Ice II, and ice III | 213 MPa | -35 °C |
| ice II, ice III, and ice V | 344 MPa | -24 °C |
| ice II, ice V, and ice VI | 626 MPa | -70 °C |
The temperature and pressure at which solid, liquid, and gaseous water coexist in equilibrium is called the triple point of water. This point is used to define the units of temperature (the kelvin and, indirectly, the degree Celsius and even the degree Fahrenheit). The triple point is at a temperature of 273.16 K (0.01 °C) by convention, and at a pressure of 611.73 Pa. This pressure is quite low, about 1/166 of the normal sea level barometric pressure of 101,325 Pa. The atmospheric surface pressure on planet Mars is remarkably close to the triple point pressure, and the zero-elevation or "sea level" of Mars is defined by the height at which the atmospheric pressure corresponds to the triple point of water.
Y-axis = Pressure in Pascal (10n),
X-axis = Temperature in Kelvin.
S = Solid
L = Liquid
V = Vapour
CP = Critical Point
TP = Triple point of water
The triple point of water (the single combination of pressure and temperature at which pure liquid water, ice, and water vapor can coexist in a stable equilibrium) is used to define the kelvin, the SI unit of thermodynamic temperature. As a consequence, water's triple point temperature is a prescribed value rather than a measured quantity: 273.16 kelvins (0.01 °C) and a pressure of 611.73 pascals (approximately 0.0060373 atm). This is approximately the combination that exists with 100% relative humidity at sea level and the freezing point of water.
Although it is commonly named as "the triple point of water", the stable combination of liquid water, ice I, and water vapour is but one of several triple points on the phase diagram of water. Gustav Heinrich Johann Apollon Tammann in Göttingen produced data on several other triple points in the early 20th century. Kamb and others documented further triple points in the 1960s.[10][9][11]
Mpemba effect
The Mpemba effect is the surprising phenomenon whereby hot water can, under certain conditions, freeze sooner than cold water, even though it must pass the lower temperature on the way to freezing. However, this can be explained with evaporation, convection, supercooling, and the insulating effect of frost.
Hot ice
Hot ice is the name given to another surprising phenomenon in which water at room temperature can be turned into ice that remains at room temperature by supplying an electric field on the order of 106 volts per meter.[12]
The effect of such electric fields has been suggested as an explanation of cloud formation. The first time cloud ice forms around a clay particle, it requires a temperature of −10 °C, but subsequent freezing around the same clay particle requires a temperature of just −5 °C, suggesting some kind of structural change.[13]
Surface tension
Water drops are stable, due to the high surface tension of water, 72.8 mN/m, the highest of the non-metallic liquids. This can be seen when small quantities of water are put on a surface such as glass: the water stays together as drops. This property is important for life. For example, when water is carried through xylem up stems in plants the strong intermolecular attractions hold the water column together. Strong cohesive properties hold the water column together, and strong adhesive properties stick the water to the xylem, and prevent tension rupture caused by transpiration pull. Other liquids with lower surface tension would have a higher tendency to "rip", forming vacuum or air pockets and rendering the xylem water transport inoperative.
Electrical properties
Pure water containing no ions is an excellent insulator, however, not even "deionized" water, is completely free of ions. Water undergoes auto-ionisation at any temperature above absolute zero. Further, because water is such a good solvent, it almost always has some solute dissolved in it, most frequently a salt. If water has even a tiny amount of such an impurity, then it can conduct electricity readily, as impurities such as salt separate into free ions in aqueous solution by which an electric current can flow.
Water can be split into its constituent elements, hydrogen and oxygen, by passing a current through it. This process is called electrolysis. Water molecules naturally dissociate into H+ and OH− ions, which are pulled toward the cathode and anode, respectively. At the cathode, two H+ ions pick up electrons and form H2 gas. At the anode, four OH− ions combine and release O2 gas, molecular water, and four electrons. The gases produced bubble to the surface, where they can be collected. It is known that the theoretical maximum electrical resistivity for water is approximately 182 kΩ·m²/m (or 18.2 MΩ·cm²/cm) at 25 °C. This figure agrees well with what is typically seen on reverse osmosis, ultrafiltered and deionized ultrapure water systems used for instance, in semiconductor manufacturing plants. A salt or acid contaminant level exceeding that of even 100 parts per trillion (ppt) in ultrapure water will begin to noticeably lower its resistivity level by up to several kilohm-square meters per meter (a change of several hundred nanosiemens per meter of conductance).
- Electrical conductivity
Pure water has a low electrical conductivity, but this increases significantly upon solvation of a small amount of ionic material water such as hydrogen chloride. Thus the risks of electrocution are much greater in water with the usual impurities not found in pure water. Any electrical properties observable in water are from the ions of mineral salts and carbon dioxide dissolved in it. Water does self-ionize where two water molecules become one hydroxide anion and one hydronium cation, but not enough to carry enough electric current to do any work or harm for most operations. In pure water, sensitive equipment can detect a very slight electrical conductivity of 0.055 µS/cm at 25 °C. Water can also be electrolyzed into oxygen and hydrogen gases but in the absence of dissolved ions this is a very slow process, as very little current is conducted. While electrons are the primary charge carriers in water (and metals), in ice (and some other electrolytes), protons are the primary carriers (see proton conductor).
Dipolar nature of water

An important feature of water is its polar nature. The water molecule forms an angle, with hydrogen atoms at the tips and oxygen at the vertex. Since oxygen has a higher electronegativity than hydrogen, the side of the molecule with the oxygen atom has a partial negative charge. A molecule with such a charge difference is called a dipole. The charge differences cause water molecules to be attracted to each other (the relatively positive areas being attracted to the relatively negative areas) and to other polar molecules. This attraction is known as hydrogen bonding, and explains many of the properties of water. Certain molecules, such as carbon dioxide, also have a difference in electronegativity between the atoms but the difference is that the shape of carbon dioxide is symmetrically aligned and so the opposing charges cancel one another out. This phenomenon of water can be seen if you hold an electrical source near a thin stream of water falling vertically, causing the stream to bend towards the electrical source.
Although hydrogen bonding is a relatively weak attraction compared to the covalent bonds within the water molecule itself, it is responsible for a number of water's physical properties. One such property is its relatively high melting and boiling point temperatures; more heat energy is required to break the hydrogen bonds between molecules. The similar compound hydrogen sulfide (H2S), which has much weaker hydrogen bonding, is a gas at room temperature even though it has twice the molecular mass of water. The extra bonding between water molecules also gives liquid water a large specific heat capacity. This high heat capacity makes water a good heat storage medium.
Hydrogen bonding also gives water its unusual behavior when freezing. When cooled to near freezing point, the presence of hydrogen bonds means that the molecules, as they rearrange to minimize their energy, form the hexagonal crystal structure of ice that is actually of lower density: hence the solid form, ice, will float in water. In other words, water expands as it freezes, whereas almost all other materials shrink on solidification.
An interesting consequence of the solid having a lower density than the liquid is that ice will melt if sufficient pressure is applied. With increasing pressure the melting point temperature drops and when the melting point temperature is lower than the ambient temperature the ice begins to melt. A significant increase of pressure is required to lower the melting point temperature —the pressure exerted by an ice skater on the ice would only reduce the melting point by approximately 0.09 °C (0.16 °F).
- Electronegative Polarity
Water has a partial negative charge (σ-) near the oxygen atom due to the unshared pairs of electrons, and partial positive charges (σ+) near the hydrogen atoms. In water, this happens because the oxygen atom is more electronegative than the hydrogen atoms — that is, it has a stronger "pulling power" on the molecule's electrons, drawing them closer (along with their negative charge) and making the area around the oxygen atom more negative than the area around both of the hydrogen atoms.
Adhesion

Water sticks to itself (cohesion) because it is polar. Water also has high adhesion properties because of its polar nature. On extremely clean/smooth glass the water may form a thin film because the molecular forces between glass and water molecules (adhesive forces) are stronger than the cohesive forces. In biological cells and organelles, water is in contact with membrane and protein surfaces that are hydrophilic; that is, surfaces that have a strong attraction to water. Irving Langmuir observed a strong repulsive force between hydrophilic surfaces. To dehydrate hydrophilic surfaces—to remove the strongly held layers of water of hydration—requires doing substantial work against these forces, called hydration forces. These forces are very large but decrease rapidly over a nanometer or less. Their importance in biology has been extensively studied by V. Adrian Parsegian of the National Institute of Health.[14] They are particularly important when cells are dehydrated by exposure to dry atmospheres or to extracellular freezing.
Surface tension

Water has a high surface tension caused by the strong cohesion between water molecules. This can be seen when small quantities of water are put onto a non-soluble surface such as polythene; the water stays together as drops. Just as significantly, air trapped in surface disturbances forms bubbles, which sometimes last long enough to transfer gas molecules to the water. Another surface tension effect is capillary waves which are the surface ripples that form from around the impact of drops on water surfaces, and some times occur with strong subsurface currents flow to the water surface. The apparent elasticity caused by surface tension drives the waves.
Capillary action
Capillary action refers to the process of water moving up a narrow tube against the force of gravity. It occurs because water adheres to the sides of the tube, and then surface tension tends to straighten the surface making the surface rise, and more water is pulled up through cohesion. The process is repeated as the water flows up the tube until there is enough water that gravity can counteract the adhesive force.
Water as a solvent
Water is also a good solvent due to its polarity. When an ionic or polar compound enters water, it is surrounded by water molecules (Hydration). The relatively small size of water molecules typically allows many water molecules to surround one molecule of solute. The partially negative dipole ends of the water are attracted to positively charged components of the solute, and vice versa for the positive dipole ends.
In general, ionic and polar substances such as acids, alcohols, and salts are relatively soluble in water, and nonpolar substances such as fats and oils are not. Nonpolar molecules stay together in water because it is energetically more favorable for the water molecules to hydrogen bond to each other than to engage in van der Waals interactions with nonpolar molecules.
An example of an ionic solute is table salt; the sodium chloride, NaCl, separates into Na+ cations and Cl- anions, each being surrounded by water molecules. The ions are then easily transported away from their crystalline lattice into solution. An example of a nonionic solute is table sugar. The water dipoles make hydrogen bonds with the polar regions of the sugar molecule (OH groups) and allow it to be carried away into solution.
- Solvation

Water is a very strong solvent, referred to as the universal solvent, dissolving many types of substances. Substances that will mix well and dissolve in water (e.g. salts) are known as "hydrophilic" (water-loving) substances, while those that do not mix well with water (e.g. fats and oils), are known as "hydrophobic" (water-fearing) substances. The ability of a substance to dissolve in water is determined by whether or not the substance can match or better the strong attractive forces that water molecules generate between other water molecules. If a substance has properties that do not allow it to overcome these strong intermolecular forces, the molecules are "pushed out" from the water, and do not dissolve. Contrary to the common misconception, water and hydrophobic substances does not "repel", and the hydration of a hydrophobic surface is energetically, but not entropically, favorable.
Amphoteric nature of water
Chemically, water is amphoteric — i.e., it is able to act as either an acid or a base. Occasionally the term hydroxic acid is used when water acts as an acid in a chemical reaction. At a pH of 7 (neutral), the concentration of hydroxide ions (OH−) is equal to that of the hydronium (H3O+) or hydrogen (H+) ions. If the equilibrium is disturbed, the solution becomes acidic (higher concentration of hydronium ions) or basic (higher concentration of hydroxide ions).
Water can act as either an acid or a base in reactions. According to the Brønsted-Lowry system, an acid is defined as a species which donates a proton (an H+ ion) in a reaction, and a base as one which receives a proton. When reacting with a stronger acid, water acts as a base; when reacting with a stronger base, it acts as an acid. For instance, it receives an H+ ion from HCl in the equilibrium:
- HCl + H2O Template:Unicode H3O+ + Cl−
Here water is acting as a base, by receiving an H+ ion.
In the reaction with ammonia, NH3, water donates an H+ ion, and is thus acting as an acid:
- NH3 + H2O Template:Unicode NH4+ + OH−
Acidity in nature
In theory, pure water has a pH of 7 at 298 K. In practice, pure water is very difficult to produce. Water left exposed to air for any length of time will rapidly dissolve carbon dioxide, forming a dilute solution of carbonic acid, with a limiting pH of about 5.7. As cloud droplets form in the atmosphere and as raindrops fall through the air minor amounts of CO2 are absorbed and thus most rain is slightly acidic. If high amounts of nitrogen and sulfur oxides are present in the air, they too will dissolve into the cloud and rain drops producing more serious acid rain problems.
Hydrogen bonding in water
A water molecule can form a maximum of four hydrogen bonds because it can accept two and donate two hydrogens. Other molecules like hydrogen fluoride, ammonia, methanol form hydrogen bonds but they do not show anomalous behaviour of thermodynamic, kinetic or structural properties like those observed in water. The answer to the apparent difference between water and other hydrogen bonding liquids lies in the fact that apart from water none of the hydrogen bonding molecules can form four hydrogen bonds either due to an inability to donate/accept hydrogens or due to steric effects in bulky residues. In water local tetrahedral order due to the four hydrogen bonds gives rise to an open structure and a 3-dimensional bonding network, which exists in contrast to the closely packed structures of simple liquids. There is a great similarity between water and silica in their anomalous behaviour, even though one (water) is a liquid which has a hydrogen bonding network while the other (silica) has a covalent network with a very high melting point. One reason that water is well suited, and chosen, by life-forms, is that it exhibits its unique properties over a temperature regime that suits diverse biological processes, including hydration.
It is believed that hydrogen bond in water is largely due to electrostatic forces and some amount of covalency. The partial covalent nature of hydrogen bond predicted by Linus Pauling in the 1930s is yet to be proven unambiguously by experiments and theoretical calculations.
Quantum properties of molecular water
Although the molecular formula of water is generally considered to be a stable result in molecular thermodynamics, recent work started in 1995 has shown that at certain scales, water may act more like H3/2O than H2O at the quantum level.[15] This result could have significant ramifications at the level of, for example, the hydrogen bond in biological, chemical and physical systems. The experiment shows that when neutrons and electrons collide with water, they scatter in a way that indicates that they only are affected by a ratio of 1.5:1 of hydrogen to oxygen respectively. However, the time-scale of this response is only seen at the level of attoseconds (10-18 seconds), and so is only relevant in highly resolved kinetic and dynamical systems.[16][17]
Heavy Water and isotopologues of water
Hydrogen has three isotopes. The most common, making up more than 95% of water, has 1 proton and 0 neutrons. A second isotope, deuterium (short form "D"), has 1 proton and 1 neutron. Deuterium, D
2O, is also known as heavy water and is used in nuclear reactors as a neutron moderator. The third isotope, tritium, has 1 proton and 2 neutrons, and is radioactive, with a half-life of 12.32 years. T
2O exists in nature only in tiny quantities, being produced primarily via cosmic ray-driven nuclear reactions in the atmosphere. D
2O is stable, but differs from H
2O in in that it is more dense - hence, "heavy water" - and in that several other physical properties are slightly different from those of common, Hydrogen-1 containing "light water". D
2O occurs naturally in ordinary water in very low concentrations. Consumption of pure isolated D
2O may affect biochemical processes - ingestion of large amounts impairs kidney and central nervous system function. However, very large amounts of heavy water must be consumed for any toxicity to be apparent, and smaller quantities can be consumed with no ill effects at all.
Transparency
Water's transparency is also an important property of the liquid. If water were not transparent, sunlight, essential to aquatic plants, would not reach into seas and oceans.
History
The properties of water have historically been used to define various temperature scales. Notably, the Kelvin, Celsius and Fahrenheit scales were, or currently are, defined by the freezing and boiling points of water. The less common scales of Delisle, Newton, Réaumur and Rømer were defined similarly. The triple point of water is a more commonly used standard point today.[18]
The first scientific decomposition of water into hydrogen and oxygen, by electrolysis, was done in 1800 by William Nicholson, an English chemist. In 1805, Joseph Louis Gay-Lussac and Alexander von Humboldt showed that water is composed of two parts hydrogen and one part oxygen (by volume).
Gilbert Newton Lewis isolated the first sample of pure heavy water in 1933.
Polywater was a hypothetical polymerized form of water that was the subject of much scientific controversy during the late 1960s. The consensus now is that it does not exist.
Pseudoscience concept is water memory.
Systematic naming
The accepted IUPAC name of water is simply "water", although there are two other systematic names which can be used to describe the molecule.
The simplest and best systematic name of water is hydrogen oxide. This is analogous to related compounds such as hydrogen peroxide, hydrogen sulfide, and deuterium oxide (heavy water). Another systematic name, oxidane, is accepted by IUPAC as a parent name for the systematic naming of oxygen-based substituent groups,[19] although even these commonly have other recommended names. For example, the name hydroxyl is recommended over oxidanyl for the –OH group. The name oxane is explicitly mentioned by the IUPAC as being unsuitable for this purpose, since it is already the name of a cyclic ether also known as tetrahydropyran in the Hantzsch-Widman system; similar compounds include dioxane and trioxane.
Systematic nomenclature and humor
Dihydrogen monoxide or DHMO is an overly pedantic systematic covalent name of water. This term has been used in parodies of chemical research that call for this "lethal chemical" to be banned. In reality, a more realistic systematic name would be hydrogen oxide, since the "di-" and "mon-" prefixes are superfluous. Hydrogen sulfide, H2S, is never referred to as "dihydrogen monosulfide", and hydrogen peroxide, H2O2, is never called "dihydrogen dioxide".
Some overzealous material safety data sheets for water list the following: Caution: May cause drowning![citation needed]
Other systematic names for water include hydroxic acid or hydroxylic acid. Likewise, the systematic alkali name of water is hydrogen hydroxide—both acid and alkali names exist for water because it is able to react both as an acid or an alkali, depending on the strength of the acid or alkali it is reacted with (amphoteric). None of these names are used widely outside of DHMO sites.
See also
- Double distilled water
- Hydrodynamics
- Viscosity of Water
- Vienna Standard Mean Ocean Water
- Water absorption of electromagnetic radiation
- Water dimer
- Water (data page)
References
- ↑ Braun, Charles L. (1993). "Why is water blue?" (HTML). J. Chem. Educ. 70 (8): 612. Unknown parameter
|coauthors=ignored (help) - ↑ Lide, D. R. (Ed.) (1990). CRC Handbook of Chemistry and Physics (70th Edn.). Boca Raton (FL):CRC Press.
- ↑ P. G. Debenedetti, P. G., and Stanley, H. E.; "Supercooled and Glassy Water", Physics Today 56 (6), p. 40–46 (2003).
- ↑ The pressure due to water vapor in the air is called the partial pressure(Dalton's law) and it is directly proportional to concentration of water molecules in air (Boyle's law).
- ↑ Adiabatic cooling resulting from the ideal gas law.
- ↑ Brown, Theodore L., H. Eugene LeMay, Jr., and Bruce E. Burston. Chemistry: The Central Science. 10th ed. Upper Saddle River, NJ: Pearson Education, Inc., 2006.
- ↑ Fine, R.A. and Millero, F.J. (1973). "Compressibility of water as a function of temperature and pressure". Journal of Chemical Physics. 59 (10): 5529. doi:10.1063/1.1679903.
- ↑ 9.0 9.1 Template:Cite paper
- ↑ Template:Cite paper
- ↑ William Cudmore McCullagh Lewis and James Rice (1922). A System of Physical Chemistry. Longmans, Green and co.
- ↑ Choi, Eun-Mi; Yoon, Young-Hwan; Lee, Sangyoub; Kang, Heon. "Freezing Transition of Interfacial Water at Room Temperature under Electric Fields". Physical Review Letters. 95 (8): 085701. doi:10.1103/PhysRevLett.95.085701.
- ↑ Connolly PJ, Saunders CPR, Gallagher MW, Bower KN, Flynn MJ, Choularton TW, Whiteway J, Lawson RP (2005). "Aircraft observations of the influence of electric fields on the aggregation of ice crystals". Quarterly Journal of the Royal Meteorological Society, Part B. 131 (608): 1695–1712. Unknown parameter
|month=ignored (help) - ↑ Physical Forces Organizing Biomolecules (PDF)
- ↑ Phil Schewe, James Riordon, and Ben Stein (31 Jul 03). "A Water Molecule's Chemical Formula is Really Not H2O". Physics News Update. Check date values in:
|date=(help) - ↑ C. A. Chatzidimitriou-Dreismann, T. Abdul Redah, R. M. F. Streffer and J. Mayers (1997). "Anomalous Deep Inelastic Neutron Scattering from Liquid H2O-D2O: Evidence of Nuclear Quantum Entanglement". Physical Review Letters. 79 (15): 2839. doi:10.1103/PhysRevLett.79.2839.
- ↑ C. A. Chatzidimitriou-Dreismann, M. Vos, C. Kleiner and T. Abdul-Redah (2003). "Comparison of Electron and Neutron Compton Scattering from Entangled Protons in a Solid Polymer". Physical Review Letters. 91 (5): 057403–4. doi:10.1103/PhysRevLett.91.057403.
- ↑ http://home.comcast.net/~igpl/Temperature.html
- ↑ Leigh, G. J. et al. 1998. Principles of chemical nomenclature: a guide to IUPAC recommendations, p. 99. Blackwell Science Ltd, UK. ISBN 0-86542-685-6
External links
- Release on the IAPWS Industrial Formulation 1997 for the Thermodynamic Properties of Water and Steam (fast computation speed)
- Release on the IAPWS Formulation 1995 for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use (simpler formulation)
- A spoof site on the "dangers" of dihydrogen monoxide
- Stockholm International Water Institute (SIWI)
- Explanation of the anomalous properties of water
- Computational Chemistry Wiki
- Water phase diagrams
- Water Absorption Spectrum
Template:WH Template:WS Template:Jb1 af:water (molekule) de:Wassermolekül la:Aqua (moleculum) scn:Acqua (elimentu) sr:Вода (молекул) fi:vesi th:น้ำ (โมเลกุล)
- Pages with citations using unsupported parameters
- CS1 maint: Multiple names: authors list
- CS1 errors: dates
- All articles with unsourced statements
- Articles with unsourced statements from October 2007
- Articles with invalid date parameter in template
- Forms of water
- Hydrides
- Hydrogen compounds
- Hydroxides
- Oxides
- Inorganic solvents
- Water chemistry
- Neutron moderators