Vemurafenib
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2];Aparna Vuppala, M.B.B.S. [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Vemurafenib is a kinase inhibitor that is FDA approved for the treatment of patients with unresectable or metastatic melanoma with [[BRAF|BRAF V600E mutation]] as detected by an FDA-approved test. Common adverse reactions include arthralgia, rash, alopecia, fatigue, photosensitivity reaction, nausea, pruritus, and skin papilloma.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Vemurafenib is indicated for the treatment of patients with unresectable or metastatic melanoma with [[BRAF|BRAF V600E mutation]] as detected by an FDA-approved test.
- Dosage: 960 mg (four 240 mg tablets) orally every 12 hours with or without a meal.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Vemurafenib in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Vemurafenib in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and efficacy of vemurafenib have not been established in pediatric patients younger than 18 years.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Vemurafenib in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Vemurafenib in pediatric patients.
Contraindications
None
Warnings
New Primary Malignancies
Cutaneous Malignancsquamous cell carcinomaies
- Cutaneous squamous cell carcinoma , keratoacanthoma, and melanoma occurred at a higher incidence in patients receiving vemurafenib compared to those in the control arm in Trial 1.
- The incidence of cutaneous squamous cell carcinomas (cuSCC) and keratoacanthomas in the vemurafenib arm was 24% compared to < 1% in the dacarbazine arm. The median time to the first appearance of cuSCC was 7 to 8 weeks; approximately 33% of patients who developed a cuSCC while receiving vemurafenib experienced at least one additional occurrence with median time between occurrences of 6 weeks. Potential risk factors associated with cuSCC observed in clinical studies using vemurafenib included age (≥ 65 years), prior skin cancer, and chronic sun exposure.
- In Trial 1, new primary malignant melanoma occurred in 2.1% (7/336) of patients receiving vemurafenib compared to none of the patients receiving dacarbazine.
- Perform dermatologic evaluations prior to initiation of therapy and every 2 months while on therapy. Manage suspicious skin lesions with excision and dermatopathologic evaluation. Consider dermatologic monitoring for 6 months following discontinuation of vemurafenib.
Non-Cutaneous Squamous Cell Carcinoma
- Non-cutaneous squamous cell carcinomas (SCC) of the head and neck can occur in patients receiving vemurafenib. Monitor patients receiving vemurafenib closely for signs or symptoms of new non-cutaneous SCC.
Other Malignancies
- Based on mechanism of action, vemurafenib may promote malignancies associated with activation of RAS through mutation or other mechanisms. Monitor patients receiving vemurafenib closely for signs or symptoms of other malignancies.
Tumor Promotion in BRAF Wild-Type Melanoma
- In vitro experiments have demonstrated paradoxical activation of MAP-kinase signaling and increased cell proliferation in BRAF wild-type cells that are exposed to BRAF inhibitors. Confirm evidence of [[BRAF|BRAF V600E mutation]] in tumor specimens prior to initiation of vemurafenib.
Hypersensitivity Reactions
- Anaphylaxis and other serious hypersensitivity reactions can occur during treatment and upon re-initiation of treatment with vemurafenib. Severe hypersensitivity reactions included generalized rash and erythema, hypotension, and drug reaction with eosinophilia and systemic symptoms (DRESS syndrome). Permanently discontinue vemurafenib in patients who experience a severe hypersensitivity reaction.
Dermatologic Reactions
Severe dermatologic reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis, can occur in patients receiving vemurafenib. Permanently discontinue vemurafenib in patients who experience a severe dermatologic reaction.
QT Prolongation
Concentration-dependent QT prolongation occurred in an uncontrolled, open-label QT sub-study in previously treated patients with BRAF V600E mutation-positive metastatic melanoma. QT prolongation may lead to an increased risk of ventricular arrhythmias, including Torsade de Pointes.
Do not start treatment in patients with uncorrectable electrolyte abnormalities, QTc > 500 ms, or long QT syndrome, or in patients who are taking medicinal products known to prolong the QT interval. Prior to and following treatment initiation or after dose modification of vemurafenib for QTc prolongation, evaluate ECG and electrolytes (including potassium, magnesium, and calcium) after 15 days, monthly during the first 3 months, and then every 3 months thereafter or more often as clinically indicated.
Withhold vemurafenib in patients who develop QTc > 500 ms (Grade 3). Upon recovery to QTc ≤ 500 ms (Grade ≤ 2), restart at a reduced dose. Permanently discontinue vemurafenib treatment if the QTc interval remains > 500 ms and increased > 60 ms from pre-treatment values after controlling cardiac risk factors for QT prolongation (e.g., electrolyte abnormalities, congestive heart failure, and bradyarrhythmias).
Hepatotoxicity
Liver injury leading to functional hepatic impairment, including coagulopathy or other organ dysfunction, can occur with vemurafenib. Monitor transaminases, alkaline phosphatase, and bilirubin before initiation of treatment and monthly during treatment, or as clinically indicated. Manage laboratory abnormalities with dose reduction, treatment interruption, or treatment discontinuation.
Concurrent Administration with Ipilimumab
The safety and effectiveness of vemurafenib in combination with Ipilimumab have not been established. In a dose-finding trial, Grade 3 increases in transaminases and bilirubin occurred in a majority of patients who received concurrent Ipilimumab (3 mg/kg) and vemurafenib (960 mg BID or 720 mg BID).
Photosensitivity
Mild to severe photosensitivity can occur in patients treated with vemurafenib. Advise patients to avoid sun exposure, wear protective clothing and use a broad spectrum UVA/UVB sunscreen and lip balm (SPF ≥ 30) when outdoors. Institute dose modifications for intolerable Grade 2 or greater photosensitivity.
Ophthalmologic Reactions
Uveitis, blurry vision, and photophobia can occur in patients treated with vemurafenib. In Trial 1, uveitis, including iritis, occurred in 2.1% (7/336) of patients receiving vemurafenib compared to no patients in the dacarbazine arm. Treatment with steroid and mydriatic ophthalmic drops may be required to manage uveitis. Monitor patients for signs and symptoms of uveitis.
Embryo-Fetal Toxicity
vemurafenib can cause fetal harm when administered to a pregnant woman based on its mechanism of action. There are no adequate and well-controlled studies in pregnant women. If this drug is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus.
Adverse Reactions
Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not predict the rates observed in a broader patient population in clinical practice.
This section describes adverse drug reactions (ADRs) identified from analyses of Trial 1 and Trial 2. Trial 1 randomized (1:1) 675 treatment-naive patients with unresectable or metastatic melanoma to receive vemurafenib 960 mg orally twice daily or dacarbazine 1000 mg/m2 intravenously every 3 weeks. In Trial 2, 132 patients with metastatic melanoma and failure of at least one prior systemic therapy received treatment with vemurafenib 960 mg orally twice daily.
TABLE 1 presents adverse reactions reported in at least 10% of patients treated with vemurafenib. The most common adverse reactions of any grade (≥ 30% in either study) in vemurafenib-treated patients were arthralgia, rash, alopecia, fatigue, photosensitivity reaction, nausea, pruritus, and skin papilloma. The most common (≥ 5%) Grade 3 adverse reactions were cuSCC and rash. The incidence of Grade 4 adverse reactions was ≤ 4% in both studies.
The incidence of adverse events resulting in permanent discontinuation of study medication in Trial 1 was 7% for the vemurafenib arm and 4% for the dacarbazine arm. In Trial 2, the incidence of adverse events resulting in permanent discontinuation of study medication was 3% in vemurafenib-treated patients. The median duration of study treatment was 4.2 months for vemurafenib and 0.8 months for dacarbazine in Trial 1, and 5.7 months for vemurafenib in Trial 2.

Clinically relevant adverse reactions reported in < 10% of patients treated with vemurafenib in the Phase 2 and Phase 3 studies include:
- Skin and subcutaneous tissue disorders: palmar-plantar erythrodysesthesia syndrome, keratosis pilaris, panniculitis, erythema nodosum, Stevens-Johnson syndrome, toxic epidermal necrolysis
- Musculoskeletal and connective tissue disorders: arthritis
- Nervous system disorders: peripheral neuropathy, VIIth nerve paralysis
- Neoplasms benign, malignant and unspecified (includes cysts and polyps): basal cell carcinoma, oropharyngeal squamous cell carcinoma
- Infections and infestations: folliculitis
- Eye disorders: retinal vein occlusion
- Vascular disorders: vasculitis
- Cardiac disorders: atrial fibrillation
TABLE 2 shows the incidence of worsening liver laboratory abnormalities in Trial 1 summarized as the proportion of patients who experienced a shift from baseline to Grade 3 or 4.

Postmarketing Experience
The following adverse reactions have been identified during postapproval use of vemurafenib. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Neoplasms benign, malignant and unspecified (incl. cysts and polyps): Progression of a pre-existing chronic myelomonocytic leukemia with NRAS mutation.
- Skin and Subcutaneous Tissue Disorders: Drug reaction with eosinophilia and systemic symptoms (DRESS syndrome).
- Blood and lymphatic systems disorder: Neutropenia
Drug Interactions
Effect of Strong CYP3A4 Inhibitors or Inducers on Vemurafenib
Vemurafenib is a substrate of CYP3A4 based on in vitro data; therefore, coadministration of strong CYP3A4 inhibitors or inducers may alter vemurafenib concentrations. Avoid coadministration of vemurafenib with strong CYP3A4 inhibitors (e.g., ketoconazole, itraconazole, clarithromycin, atazanavir, nefazodone, saquinavir, telithromycin, ritonavir, indinavir, nelfinavir, voriconazole) or strong inducers (e.g., phenytoin, carbamazepine, rifampin, rifabutin, rifapentine, phenobarbital), and replace these drugs with alternative drugs when possible.
Effect of Vemurafenib on CYP1A2 Substrates
Concomitant use of vemurafenib with drugs with a narrow therapeutic window that are predominantly metabolized by CYP1A2 is not recommended as vemurafenib may increase concentrations of CYP1A2 substrates. If coadministration cannot be avoided, monitor closely for toxicities and consider a dose reduction of concomitant CYP1A2 substrates.
Ipilimumab
Increases in transaminases and bilirubin occurred in a majority of patients who received concurrent Ipilimumab and vemurafenib.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): D Vemurafenib can cause fetal harm when administered to a pregnant woman based on its mechanism of action.
Vemurafenib revealed no evidence of teratogenicity in rat embryo/fetuses at doses up to 250 mg/kg/day (approximately 1.3 times the human clinical exposure based on AUC) or rabbit embryo/fetuses at doses up to 450 mg/kg/day (approximately 0.6 times the human clinical exposure based on AUC). Fetal drug levels were 3–5% of maternal levels, indicating that vemurafenib has the potential to be transmitted from the mother to the developing fetus. There are no adequate and well controlled studies in pregnant women. Women of childbearing potential and men should be advised to use appropriate contraceptive measures during vemurafenib therapy and for at least 2 months after discontinuation of vemurafenib. If this drug is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Vemurafenib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Vemurafenib during labor and delivery.
Nursing Mothers
It is not known whether vemurafenib is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions from vemurafenib in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and efficacy in pediatric patients below the age of 18 have not been established.
Geriatic Use
Clinical studies of vemurafenib did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
Gender
Based on the population pharmacokinetic analysis gender do not have a clinically important effect on the exposure of vemurafenib.
Race
There are insufficient data to evaluate potential differences in the pharmacokinetics of vemurafenib by race.
Renal Impairment
No formal clinical study has been conducted to evaluate the effect of renal impairment on the pharmacokinetics of vemurafenib. No dose adjustment is recommended for patients with mild and moderate renal impairment based on a population pharmacokinetic analysis. The appropriate dose of vemurafenib has not been established in patients with severe renal impairment.
Hepatic Impairment
No formal clinical study has been conducted to evaluate the effect of hepatic impairment on the pharmacokinetics of vemurafenib. No dose adjustment is recommended for patients with mild and moderate hepatic impairment based on a population pharmacokinetic analysis. The appropriate dose of vemurafenib has not been established in patients with severe hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Vemurafenib in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Vemurafenib in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Vemurafenib Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Vemurafenib and IV administrations.
Overdosage
There is no information on overdosage of vemurafenib.
Pharmacology
| |
Vemurafenib
| |
| Systematic (IUPAC) name | |
| N-(3-{[5-(4-chlorophenyl)-1H-pyrrolo[2,3-b]pyridin-3-yl]carbonyl}-2,4-difluorophenyl)propane-1-sulfonamide | |
| Identifiers | |
| CAS number | |
| ATC code | L01 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 489.92 g/mol |
| SMILES | & |
| Synonyms | PLX4032, RG7204, RO5185426 |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Licence data |
, |
| Pregnancy cat. | |
| Legal status |
Prescription Only (S4)(AU) ?(CA) POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral |
Template:Infobox drug mechanism
Mechanism of Action
Vemurafenib is a low molecular weight, orally available inhibitor of some mutated forms of BRAF serine-threonine kinase, including BRAF V600E. Vemurafenib also inhibits other kinases in vitro such as CRAF, ARAF, wild-type BRAF, SRMS, ACK1, MAP4K5, and FGR at similar concentrations. Some mutations in the BRAF gene including V600E result in constitutively activated BRAF proteins, which can cause cell proliferation in the absence of growth factors that would normally be required for proliferation. Vemurafenib has anti-tumor effects in cellular and animal models of melanomas with mutated BRAF V600E.
Structure
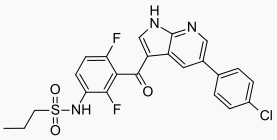
Vemurafenib has the chemical name propane-1-sulfonic acid {3-[5-(4-chlorophenyl)-1H-pyrrolo[2,3-b]pyridine-3-carbonyl]-2,4-difluoro-phenyl}-amide. It has the molecular formula C23H18ClF2N3O3S and a molecular weight of 489.9. Vemurafenib has the following chemical structure:

Pharmacodynamics
Cardiac Electrophysiology
In a multi-center, open-label, single-arm study in 132 patients with BRAF V600E mutation-positive metastatic melanoma, patients administered vemurafenib 960 mg orally twice daily did not experience large changes in mean QTc interval (i.e., > 20 ms) from baseline. Vemurafenib is associated with concentration-dependent QTc interval prolongation. The largest mean change from baseline in the first month of treatment occurred at 2 hours post-dose on Day 15—an increase of 12.8 ms (upper boundary of the two-sided 90% confidence interval of 14.9 ms). In the first 6 months of treatment, the largest observed mean change from baseline occurred at a pre-dose time point—an increase of 15.1 ms (upper boundary of the two-sided 90% confidence interval of 17.7 ms).
Pharmacokinetics
The pharmacokinetics of vemurafenib were determined in patients with BRAF mutation-positive metastatic melanoma following 15 days of 960 mg twice daily with dosing approximately 12 hours apart. The population pharmacokinetic analysis pooled data from 458 patients. At steady-state, vemurafenib exhibits linear pharmacokinetics within the 240 mg to 960 mg dose range.
Absorption
The bioavailability of vemurafenib has not been determined. The median Tmax was approximately 3 hours following multiple doses.
The mean (± SD) Cmax and AUC0-12 were 62 ± 17 µg/mL and 601 ± 170 µg*h/mL, respectively. The median accumulation ratio estimate from the population pharmacokinetic analysis for the twice daily regimen is 7.4, with steady-state achieved at approximately 15 to 22 days.
In clinical trials, vemurafenib was administered without regard to food. A food effect study has demonstrated that a single dose of vemurafenib administered with a high-fat meal increased AUC by approximately 5-fold, increased Cmax by 2.5-fold, and delayed Tmax by approximately 4 hours as compared to the fasted state.
QTc prolongation may occur with increased exposures as vemurafenib is associated with concentration-dependent QTc interval prolongation.
Distribution
Vemurafenib is highly bound (> 99%) to human albumin and alpha-1 acid glycoprotein plasma proteins. The population apparent volume of distribution is estimated to be 106 L (with 66% inter-patient variability).
Metabolism
Following oral administration of 960 mg of 14C-vemurafenib, mean data showed that vemurafenib and its metabolites represented 95% and 5% of the components in plasma over 48 hours, respectively.
Elimination
Following oral administration of 960 mg of 14C-vemurafenib, approximately 94% of the radioactive dose was recovered in feces and approximately 1% was recovered in the urine. The population apparent clearance is estimated to be 31 L/day (with 32% inter-patient variability). The median elimination half-life estimate for vemurafenib is 57 hours (the 5th and 95th percentile range is 30 to 120 hours).
Nonclinical Toxicology
Carcinogenesis and Mutagenesis
There have been no formal studies conducted assessing the carcinogenic potential of vemurafenib. vemurafenib increased the development of cutaneous squamous cell carcinomas in patients in clinical trials.
Vemurafenib did not cause genetic damage when tested in in vitro assays (bacterial mutation [AMES Assay], human lymphocyte chromosome aberration) or in the in vivo rat bone marrow micronucleus test.
Animal Toxicology and/or Pharmacology
Consistent with the increased incidence of cutaneous squamous cell carcinomas in patients treated with vemurafenib, the treatment of mice implanted with human cuSCC cells with vemurafenib caused a dose dependent acceleration of the growth of the implanted tumors.
Clinical Studies
Treatment Naive Patients
Trial 1, an international, open-label, randomized controlled trial, equally allocated 675 patients with treatment-naive, BRAF V600E mutation-positive unresectable or metastatic melanoma, as detected by the cobas® 4800 BRAF V600 Mutation Test, to receive vemurafenib 960 mg by mouth twice daily (n=337) or dacarbazine 1000 mg/m2 intravenously on Day 1 every 3 weeks (n=338). Randomization stratification factors were disease stage, lactate dehydrogenase (LDH), ECOG performance status, and geographic region. Treatment continued until disease progression, unacceptable toxicity, and/or consent withdrawal. The major efficacy outcome measures of the trial were overall survival (OS) and investigator-assessed progression-free survival (PFS). Other outcome measures included confirmed investigator-assessed best overall response rate.
Baseline characteristics were balanced between treatment groups. Most patients were male (56%) and caucasian (99%), the median age was 54 years (24% were ≥ 65 years), all patients had ECOG performance status of 0 or 1, and the majority of patients had metastatic disease (95%).
Trial 1 demonstrated statistically significant increases in overall survival and progression-free survival in the vemurafenib arm compared to the dacarbazine control arm. TABLE 3 and FIGURE 1 summarize the efficacy results.

The confirmed, investigator-assessed best overall response rate was 48.4% (95% CI: 41.6%, 55.2%) in the vemurafenib arm compared to 5.5% (95% CI: 2.8%, 9.3%) in the dacarbazine arm. There were 2 complete responses (0.9%) and 104 partial responses (47.4%) in the vemurafenib arm and all 12 responses were partial responses (5.5%) in the dacarbazine arm.
Patients Who Received Prior Systemic Therapy
In a single-arm, multicenter, multinational trial (Trial 2), 132 patients with BRAF V600E mutation-positive metastatic melanoma, as detected by the cobas® 4800 BRAF V600 Mutation Test, who had received at least one prior systemic therapy, received vemurafenib 960 mg by mouth twice daily. The median age was 52 years with 19% of patients being older than 65 years. The majority of patients were male (61%) and Caucasian (99%). Forty-nine percent of patients received ≥ 2 prior therapies. The median duration of follow-up was 6.87 months (range, 0.6 to 11.3).
The confirmed best overall response rate as assessed by an independent review committee (IRC) was 52% (95% CI: 43%, 61%). There were 3 complete responses (2.3%) and 66 partial responses (50.0%). The median time to response was 1.4 months with 75% of responses occurring by month 1.6 of treatment. The median duration of response by IRC was 6.5 months (95% CI: 5.6, not reached).
Patients with Wild-Type BRAF Melanoma
Vemurafenib has not been studied in patients with wild-type BRAF melanoma.
How Supplied
Vemurafenib is supplied as 240 mg film-coated tablets:
- NDC 50242-090-01 single bottle of 120 count
- NDC 50242-090-02 single bottle of 112 count
Storage
Store at room temperature 20°C–25°C (68°F–77°F)
Images
Drug Images
{{#ask: Page Name::Vemurafenib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Vemurafenib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Health care providers should advise patients of the potential benefits and risks of vemurafenib and instruct their patients to read the MEDICATION GUIDE before starting vemurafenib therapy. Inform patients of the following:
- Evidence of BRAF V600E mutation in the tumor specimen with an FDA approved test is necessary to identify patients for whom treatment with vemurafenib is indicated.
- vemurafenib increases the risk of developing new primary cutaneous malignancies. Advise patients of the importance of contacting their health care provider immediately for any changes in their skin.
- Anaphylaxis and other serious hypersensitivity reactions can occur during treatment and upon re-initiation of treatment with vemurafenib. Advise patients to stop taking vemurafenib and to seek immediate medical attention for symptoms of anaphylaxis or hypersensitivity.
- Severe dermatologic reactions can occur in patients receiving vemurafenib. Advise patients to stop taking vemurafenib and to contact their health care provider for severe dermatologic reactions.
- Vemurafenib can prolong QT interval, which may result in ventricular arrhythmias. Advise patients of the importance of monitoring of their electrolytes and the electrical activity of their heart (via an ECG) during vemurafenib treatment .
- Liver injury leading to functional hepatic impairment, including coagulopathy or other organ dysfunction, can occur with vemurafenib. Advise patients of the importance of laboratory monitoring of their liver during vemurafenib treatment and to contact their health care provider for relevant symptoms.
- Vemurafenib can cause mild to severe photosensitivity. Advise patients to avoid sun exposure, wear protective clothing, and use a broad spectrum UVA/UVB sunscreen and lip balm (SPF ≥ 30) when outdoors to help protect against sunburn.
- Ophthalmologic reactions can occur in patients treated with vemurafenib. Advise patients to contact their health care provider immediately for ophthalmologic symptoms.
- Vemurafenib can cause fetal harm when administered to a pregnant woman based on its mechanism of action. Advise women of childbearing potential and men to use appropriate contraceptive measures during vemurafenib therapy and for at least 2 months after discontinuation of vemurafenib. Advise patients to contact their health care provider immediately if they become pregnant.
Precautions with Alcohol
Alcohol-Vemurafenib interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Zelboraf [1]
Look-Alike Drug Names
There is limited information regarding Vemurafenib Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Vemurafenib |Label Name=Vemurafenib 240 mg.png
}}