Streptozocin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning
See full prescribing information for complete Boxed Warning.
|
Overview
Streptozocin is an alkylating agent, antineoplastic agent and nitrosourea that is FDA approved for the treatment of metastatic islet cell carcinoma of the pancreas. There is a Black Box Warning for this drug as shown here. Common adverse reactions include nausea, confusion, lethargy and depression.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Streptozocin sterile powder should be administered intravenously by rapid injection or short/prolonged infusion. It is not active orally. Although it has been administered intraarterially, this is not recommended pending further evaluation of the possibility that adverse renal effects may be evoked more rapidly by this route of administration.
Two different dosage schedules have been employed successfully with Streptozocin.
Daily Schedule
- The recommended dose for daily intravenous administration is 500 mg/m2 of body surface area for five consecutive days every six weeks until maximum benefit or until treatment-limiting toxicity is observed. Dose escalation on this schedule is not recommended.
Weekly Schedule
- The recommended initial dose for weekly intravenous administration is 1000 mg/m2 of body surface area at weekly intervals for the first two courses (weeks). In subsequent courses, drug doses may be escalated in patients who have not achieved a therapeutic response and who have not experienced significant toxicity with the previous course of treatment. However, A SINGLE DOSE OF 1500 mg/m2 BODY SURFACE AREA SHOULD NOT BE EXCEEDED as a greater dose may cause azotemia. When administered on this schedule, the median time to onset of response is about 17 days and the median time to maximum response is about 35 days. The median total dose to onset of response is about 2000 mg/m2 body surface area and the median total dose to maximum response is about 4000 mg/m2 body surface area.
- The ideal duration of maintenance therapy with Streptozocin has not yet been clearly established for either of the above schedules.
- For patients with functional tumors, serial monitoring of fasting insulin levels allows a determination of biochemical response to therapy. For patients with either functional or nonfunctional tumors, response to therapy can be determined by measurable reductions of tumor size (reduction of organomegaly, masses, or lymph nodes).
- Reconstitute Streptozocin with 9.5 mL of dextrose injection, USP, or 0.9% sodium chloride injection, USP. The resulting pale-gold solution will contain 100 mg of streptozocin and 22 mg of citric acid per mL. Where more dilute infusion solutions are desirable, further dilution in the above vehicles is recommended. The total storage time for streptozocin after it has been placed in solution should not exceed 12 hours. This product contains no preservatives and is not intended as a multiple-dose vial.
Caution in the handling and preparation of the powder and solution should be exercised, and the use of gloves is recommended. If the sterile powder of Streptozocin or a solution prepared from Streptozocin contacts the skin or mucosae, immediately wash the affected area with soap and water. Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published.1–7 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Streptozocin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Streptozocin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Streptozocin FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Streptozocin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Streptozocin in pediatric patients.
Contraindications
There is limited information regarding Streptozocin Contraindications in the drug label.
Warnings
|
Warning
See full prescribing information for complete Boxed Warning.
|
Renal Toxicity
- Many patients treated with Streptozocin have experienced renal toxicity, as evidenced by azotemia, anuria, hypophosphatemia, glycosuria and renal tubular acidosis. Such toxicity is dose-related and cumulative and may be severe or fatal.
- Renal function must be monitored before and after each course of therapy. Serial urinalysis, blood urea nitrogen, plasma creatinine, serum electrolytes and creatinine clearance should be obtained prior to, at least weekly during, and for four weeks after drug administration. Serial urinalysis is particularly important for the early detection of proteinuria and should be quantitated with a 24 hour collection when proteinuria is detected. Mild proteinuria is one of the first signs of renal toxicity and may herald further deterioration of renal function. Reduction of the dose of Streptozocin or discontinuation of treatment is suggested in the presence of significant renal toxicity. Adequate hydration may help reduce the risk of nephrotoxicity to renal tubular epithelium by decreasing renal and urinary concentration of the drug and its metabolites.
- Use of Streptozocin in patients with preexisting renal disease requires a judgment by the physician of potential benefit as opposed to the known risk of serious renal damage.
- This drug should not be used in combination with or concomitantly with other potential nephrotoxins.
- When exposed dermally, some rats developed benign tumors at the site of application of streptozocin. Consequently, streptozocin may pose a carcinogenic hazard following topical exposure if not properly handled.
Adverse Reactions
Clinical Trials Experience
Renal
- See warnings.
Gastrointestinal
- Most patients treated with Streptozocin have experienced severe nausea and vomiting, occasionally requiring discontinuation of drug therapy. Some patients experienced diarrhea. A number of patients have experienced hepatic toxicity, as characterized by elevated liver enzyme (SGOT and LDH) levels and hypoalbuminemia.
Hematological
- Hematological toxicity has been rare, most often involving mild decreases in hematocrit values. However, fatal hematological toxicity with substantial reductions in leukocyte (Leukopenia) and platelet (Thrombocytopenia) count has been observed.
Metabolic
- Mild to moderate abnormalities of glucose tolerance have been noted in some patients treated with Streptozocin. These have generally been reversible, but insulin shock with hypoglycemia has been observed.
Genitourinary
- Two cases of nephrogenic diabetes insipidus following therapy with Streptozocin have been reported. One had spontaneous recovery and the second responded to indomethacin.
Postmarketing Experience
- Spontaneous reports have been received of local inflammation (i.e., edema, erythema, burning, tenderness) following extravasation of the product. In most cases, these events resolved the same day or within a few days.
Drug Interactions
There is limited information regarding Streptozocin Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Reproduction studies revealed that streptozocin is teratogenic in the rat and has abortifacient effects in rabbits. When administered intravenously to pregnant monkeys, it appears rapidly in the fetal circulation. There are no studies in pregnant women. Streptozocin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Streptozocin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Streptozocin during labor and delivery.
Nursing Mothers
- It is not known whether streptozocin is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants, nursing should be discontinued in patients receiving Streptozocin.
Pediatric Use
There is no FDA guidance on the use of Streptozocin in pediatric settings.
Geriatic Use
- Clinical studies of streptozocin did not include sufficient numbers of patients aged 65 years and older to determine whether there was a difference in either efficacy or toxicity as compared to younger patients. Other reported clinical experience has not identified differences in efficacy or safety between the elderly and younger patient populations. In general, dose selection for elderly patients should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Streptozocin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Streptozocin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Streptozocin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Streptozocin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Streptozocin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Streptozocin in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Streptozocin Administration in the drug label.
Monitoring
There is limited information regarding Streptozocin Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Streptozocin and IV administrations.
Overdosage
No specific antidote for Streptozocin is known.
Pharmacology
| |
Streptozocin
| |
| Systematic (IUPAC) name | |
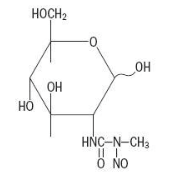
| 2-Deoxy-2-({[methyl(nitroso)amino]carbonyl}amino)-β-D-glucopyranose | |
| Identifiers | |
| CAS number | |
| ATC code | L01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 265.221 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 17-25% |
| Metabolism | liver, kidney |
| Half life | 35-40 minutes |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
D(US) |
| Legal status |
Rx only |
| Routes | Intravenous |
Mechanism of Action
- Streptozocin inhibits DNA synthesis in bacterial and mammalian cells. In bacterial cells, a specific interaction with cytosine moieties leads to degradation of DNA. The biochemical mechanism leading to mammalian cell death has not been definitely established; streptozocin inhibits cell proliferation at a considerably lower level than that needed to inhibit precursor incorporation into DNA or to inhibit several of the enzymes involved in DNA synthesis. Although streptozocin inhibits the progression of cells into mitosis, no specific phase of the cell cycle is particularly sensitive to its lethal effects.
Structure
The structural formula is represented below:

Pharmacodynamics
There is limited information regarding Streptozocin Pharmacodynamics in the drug label.
Pharmacokinetics
- Streptozocin is active in the L1210 leukemic mouse over a fairly wide range of parenteral dosage schedules. In experiments in many animal species, streptozocin induced a diabetes that resembles human hyperglycemic nonketotic diabetes mellitus. This phenomenon, which has been extensively studied, appears to be mediated through a lowering of beta cell nicotinamide adenine dinucleotide (NAD) and consequent histopathologic alteration of pancreatic islet beta cells.
- The metabolism and the chemical dissociation of streptozocin that occurs under physiologic conditions has not been extensively studied. When administered intravenously to a variety of experimental animals, streptozocin disappears from the blood very rapidly. In all species tested, it was found to concentrate in the liver and kidney. As much as 20% of the drug (or metabolites containing an N-nitrosourea group) is metabolized and/or excreted by the kidney. Metabolic products have not yet been identified.
Nonclinical Toxicology
There is limited information regarding Streptozocin Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Streptozocin Clinical Studies in the drug label.
How Supplied

Storage
Unopened vials of Streptozocin should be stored at refrigeration temperatures (2° to 8°C) and protected from light (preferably stored in carton).
Images
Drug Images
{{#ask: Page Name::Streptozocin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

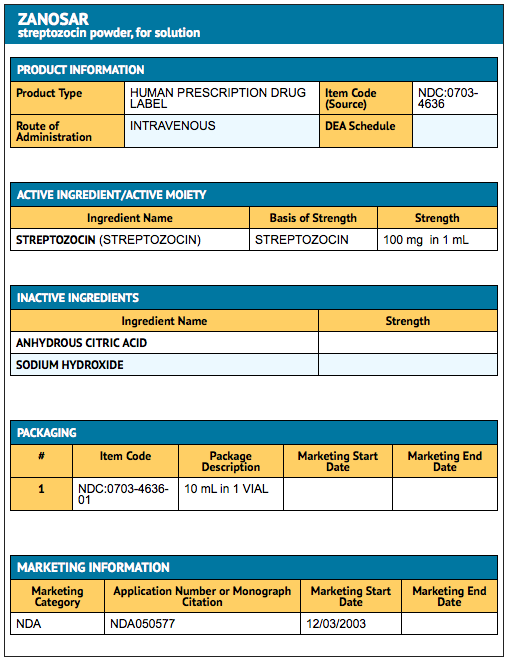
{{#ask: Label Page::Streptozocin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Streptozocin Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Streptozocin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Streptozocin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.