Polythiazide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]; Adeel Jamil, M.D. [3]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Polythiazide is a thiazide diuretic that is FDA approved for the treatment of edema associated with congestive heart failure, hepatic cirrhosis, and corticosteroid and estrogen therapy and various forms of renal dysfunction; and hypertension.. Common adverse reactions include cardiac dysrhythmia, hypotension, disorder of glucose regulation, hypercalcemia, hyperuricemia, hypokalemia, hypomagnesemia hyponatremia, immune hypersensitivity reaction, systemic lupus erythematosus.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Dosing Information
- Usual dosage: 1-4 mg PO qd
Antihypertensive
- Dosing Information
- Usual dosage: 2-4 mg PO qd
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Polythiazide in adult patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Polythiazide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Polythiazide in pediatric patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Polythiazide in pediatric patients.
Contraindications
Anuria. Hypersensitivity to this or other sulfonamide derived drugs.
Warnings
Thiazides should be used with caution in severe renal disease. In patients with renal disease, thiazides may precipitate azotemia. Cumulative effects of the drug may develop in patients with impaired renal function. Thiazides should be used with caution in patients with impaired hepatic function or progressive liver disease, since minor alterations of fluid and electrolyte balance may precipitate hepatic coma. Thiazides may add to or potentiate the action of other antihypertensive drugs. Potentiation occurs with ganglionic or peripheral adrenergic blocking drugs. Sensitivity reactions may occur in patients with a history of allergy or bronchial asthma. The possibility of exacerbation or activation of systemic lupus erythematosus has been reported.
PRECAUTIONS
Periodic determination of serum electrolytes to detect possible electrolyte imbalance should be performed at appropriate intervals. All patients receiving thiazide therapy should be observed for clinical signs of fluid or electrolyte imbalance; namely, hyponatremia, hypochloremic alkalosis, and hypokalemia. Serum and urine electrolyte determinations are particularly important when the patient is vomiting excessively or receiving parenteral fluids. Medication such as digitalis may also influence serum electrolytes. Warning signs, irrespective of cause, are: dryness of mouth, thirst, weakness, lethargy, drowsiness, restlessness, muscle pains or cramps, muscular fatigue, hypotension, oliguria, tachycardia, and gastrointestinal disturbances such as nausea and vomiting. Hypokalemia may develop with thiazides as with any other potent diuretic, especially with brisk diuresis, when severe cirrhosis is present, or during concomitant use of corticosteroids or ACTH. Interference with adequate oral electrolyte intake will also contribute to hypokalemia. Digitalis therapy may exaggerate metabolic effects of hypokalemia especially with reference to myocardial activity. Any chloride deficit is generally mild and usually does not require specific treatment except under extraordinary circumstances (as in liver disease or renal disease). Dilutional hyponatremia may occur in edematous patients in hot weather; appropriate therapy is water restriction, rather than administration of salt except in rare instances when the hyponatremia is life threatening. In actual salt depletion, appropriate replacement is the therapy of choice. Hyperuricemia may occur or frank gout may be precipitated in certain patients receiving thiazide therapy. Insulin requirements in diabetic patients may be increased, decreased, or unchanged. Latent diabetes mellitus may become manifest during thiazide administration. Thiazide drugs may increase the responsiveness to tubocurarine. The antihypertensive effects of the drug may be enhanced in the postsympathectomy patient. Thiazides may decrease arterial responsiveness to norepinephrine. This diminution is not sufficient to preclude effectiveness of the pressor agent for therapeutic use. If progressive renal impairment becomes evident, as indicated by a rising nonprotein nitrogen or blood urea nitrogen, a careful reappraisal of therapy is necessary with consideration given to withholding or discontinuing diuretic therapy. Thiazides may decrease serum PBI levels without signs of thyroid disturbance.
Adverse Reactions
Clinical Trials Experience
- Gastrointestinal System Reactions
- Central Nervous System Reactions
- Hematologic Reactions
- Dermatologic—Hypersensitivity Reactions
- Cardiovascular Reaction
- Orthostatic hypotension may occur and may be aggravated by alcohol, barbiturates or narcotics.
- F. Other
Whenever adverse reactions are moderate or severe, thiazide dosage should be reduced or therapy withdrawn.
Postmarketing Experience
There is limited information about the post marketing experience.
Drug Interactions
There is no FDA guidance on the use of Polythiazide with respect to specific drug interactions.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
Thiazides cross the placental barrier and appear in cord blood. The use of thiazides in pregnant women requires that the anticipated benefit be weighed against possible hazards to the fetus. These hazards include fetal or neonatal jaundice, thrombocytopenia, and possibly other adverse reactions which have occurred in the adult.
Pregnancy Category (AUS):
Thiazides appear in breast milk. If use of the drug is deemed essential, the patient should stop nursing.
Labor and Delivery
There is no FDA guidance on use of Polythiazide during labor and delivery.
Nursing Mothers
Thiazides appear in breast milk. If use of the drug is deemed essential, the patient should stop nursing.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
There is no FDA guidance on the use of Polythiazide in geriatric settings.
Gender
There is no FDA guidance on the use of Polythiazide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Polythiazide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Polythiazide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Polythiazide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Polythiazide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Polythiazide in patients who are immunocompromised.
Administration and Monitoring
Administration
Therapy should be individualized according to patient response. This therapy should be titrated to gain maximal therapeutic response as well as the minimal dose possible to maintain that therapeutic response. The usual dosage of Renese tablets for diuretic therapy is 1 to 4 mg daily, and for antihypertensive therapy is 2 to 4 mg daily.
Monitoring
There is limited information about the drug monitoring.
IV Compatibility
There is limited information about the IV Compatibility.
Overdosage
There is limited information regarding Polythiazide overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
| |
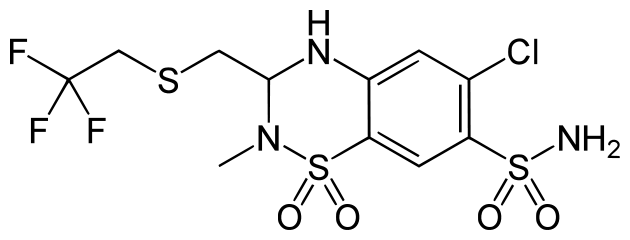
Polythiazide
| |
| Systematic (IUPAC) name | |
| 6-chloro-2-methyl-3-{[(2,2,2-trifluoroethyl)thio]methyl}-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide | |
| Identifiers | |
| CAS number | |
| ATC code | C03 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 439.88 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
The mechanism of action results in an interference with the renal tubular mechanism of electrolyte reabsorption. At maximal therapeutic dosage all thiazides are approximately equal in their diuretic potency. The mechanism whereby thiazides function in the control of hypertension is unknown.
Structure

Pharmacodynamics
There is no FDA information on the use of Polythiazide with respect to pharmacodynamics.
Pharmacokinetics
There is no FDA information on the use of Polythiazide with respect to pharmacokinetics.
Nonclinical Toxicology
There is no FDA information on the use of Polythiazide with respect to nonclinical toxicology.
Clinical Studies
There is limited information regarding Polythiazide Clinical Studies in the drug label.
How Supplied
RENESE (polythiazide) Tablets are available as: 1 mg white, scored tablets in bottles of 100 (NDC 0069-3750-66). 2 mg yellow, scored tablets in bottles of 100 (NDC 0069-3760-66). 4 mg white, scored tablets in bottles of 100 (NDC 0069-3770-66).
Storage
There is limited information regarding Polythiazide Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Polythiazide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Polythiazide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is no FDA guidance on the use of Polythiazide with respect to FDA Provided Patient Information.
Precautions with Alcohol
Alcohol-Polythiazide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Polythiazide Brand Names in the drug label.
Look-Alike Drug Names
There is limited information about the Look-Alike Drug Names.
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Polythiazide |Pill Name=No_image.jpg |Drug Name=Polythiazide |Pill Ingred=dibasic calcium phosphate, lactose, magnesium stearate, polyethylene glycol, sodium lauryl sulfate, starch, vanillin|+sep=; |Pill Imprint=Pfizer;375 |Pill Dosage=1 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=7.00 |Pill Scoring=2 |Pill Image= |Drug Author=Pfizer Labs |NDC=0069-3750-66
}}
{{#subobject:
|Page Name=Polythiazide |Pill Name=No_image.jpg |Drug Name=Polythiazide |Pill Ingred=dibasic calcium phosphate, lactose, magnesium stearate, polyethylene glycol, sodium lauryl sulfate, starch, vanillin|+sep=; |Pill Imprint=Pfizer;376 |Pill Dosage=2 mg |Pill Color=Yellow|+sep=; |Pill Shape=Round |Pill Size (mm)=9.00 |Pill Scoring=2 |Pill Image= |Drug Author=Pfizer Labs |NDC=0069-3760-66
}}
{{#subobject:
|Page Name=Polythiazide |Pill Name=No_image.jpg |Drug Name=Polythiazide |Pill Ingred=dibasic calcium phosphate, lactose, magnesium stearate, polyethylene glycol, sodium lauryl sulfate, starch, vanillin|+sep=; |Pill Imprint=Pfizer;377 |Pill Dosage=4 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=9.00 |Pill Scoring=2 |Pill Image= |Drug Author=Pfizer Labs |NDC=0069-3770-66
}}
{{#subobject:
|Label Page=Polythiazide |Label Name=Polythiazide_label_01.png
}}
{{#subobject:
|Label Page=Polythiazide |Label Name=Polythiazide_panel_01.png
}}
{{#subobject:
|Label Page=Polythiazide |Label Name=Polythiazide_panel_02.jpg
}}
{{#subobject:
|Label Page=Polythiazide |Label Name=Polythiazide_panel_03.jpg
}}