Oxandrolone
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
|
Overview
Oxandrolone is an anabolic steroid that is FDA approved for the {{{indicationType}}} of weight loss following extensive surgery, chronic infections, or severe trauma, and in some patients who without definite pathophysiologic reasons fail to gain or to maintain normal weight, to offset the protein catabolism associated with prolonged administration of corticosteroids, and for the relief of the bone pain frequently accompanying osteoporosis. There is a Black Box Warning for this drug as shown here. Common adverse reactions include edema, increased risk of atherosclerosis, cholestatic hepatitis, jaundice, testicular atropy, erectile dysfunction, and priapism.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Weight Loss
- Oxandrolone is indicated as adjunctive therapy to promote weight gain after weight loss following extensive surgery, chronic infections, or severe trauma, and in some patients who without definite pathophysiologic reasons fail to gain or to maintain normal weight, to offset the protein catabolism associated with prolonged administration of corticosteroids, and for the relief of the bone pain frequently accompanying osteoporosis.
- Therapy with anabolic steroids is adjunctive to and not a replacement for conventional therapy. The duration of therapy with oxandrolone will depend on the response of the patient and the possible appearance of adverse reactions. Therapy should be intermittent.
- The response of individuals to anabolic steroids varies. The daily adult dosage is 2.5 mg to 20 mg given in 2 to 4 divided doses. The desired response may be achieved with as little as 2.5 mg or as much as 20 mg daily. A course of therapy of 2 to 4 weeks is usually adequate. This may be repeated intermittently as indicated.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Oxandrolone in adult patients.
Non–Guideline-Supported Use
Alcoholic hepatitis
- Oxandrolone (80 milligrams daily).[1]
Burn, Severe; Adjunct
- Oxandrolone 20 mg/day in 2 divided doses.[2]
Cachexia associated with AIDS
- Oxandrolone 20 milligrams.[3]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Weight Loss
- The total daily dosage of oxandrolone is ≤0.1 mg per kilogram body weight or ≤0.045 mg per pound of body weight. This may be repeated intermittently as indicated.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Oxandrolone in pediatric patients.
Non–Guideline-Supported Use
Burn, Severe; Adjunct
- Oxandrolone 0.1 mg/kg orally twice daily.[4]
Turner syndrome
- Oral oxandrolone in usual dosages of 0.125 mg/kg/day for 1 to 2 years.[5]
Contraindications
- Known or suspected prostate cancer or the male breast.
- Breast cancer in females with hypercalcemia (androgenic anabolic steroids may stimulate osteolytic bone resorption).
- Pregnancy, because of possible masculinization of the fetus. Oxandrolone has been shown to cause embryotoxicity, fetotoxicity, infertility, and masculinization of female animal offspring when given in doses 9 times the human dose.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
|
- Cholestatic hepatitis and jaundice may occur with 17-alpha-alkylated androgens at a relatively low dose. If cholestatic hepatitis with jaundice appears or if liver function tests become abnormal, oxandrolone should be discontinued and the etiology should be determined. Drug-induced jaundice is reversible when the medication is discontinued.
- In patients with breast cancer, anabolic steroid therapy may cause hypercalcemia by stimulating osteolysis. Oxandrolone therapy should be discontinued if hypercalcemia occurs.
- Edema with or without congestive heart failure may be a serious complication in patients with pre-existing cardiac, renal, or hepatic disease. Concomitant administration of adrenal cortical steroid or ACTH may increase the edema.
- In children, androgen therapy may accelerate bone maturation without producing compensatory gain in linear growth. This adverse effect results in compromised adult height. The younger the child, the greater the risk of compromising final mature height. The effect on bone maturation should be monitored by assessing bone age of the left wrist and hand every 6 months.
- Geriatric patients treated with androgenic anabolic steroids may be at an increased risk for the development of prostatic hypertrophy and prostatic carcinoma.
- Anabolic steroids have not been shown to enhance athletic ability.
Precautions
- Concurrent dosing of oxandrolone with warfarin may result in unexpectedly large increases in the INR or prothrombin time (PT). When oxandrolone is prescribed to patients being treated with warfarin, doses of warfarin may need to be decreased significantly to maintain the desirable INR level and diminish the risk of potentially serious bleeding.
- General
- Women should be observed for signs of virilization (deepening of the voice, hirsutism, acne, clitoromegaly). Discontinuation of drug therapy at the time of evidence of mild virilism is necessary to prevent irreversible virilization. Some virilizing changes in women are irreversible even after prompt discontinuance of therapy and are not prevented by concomitant use of estrogens. Menstrual irregularities may also occur.
- Anabolic steroids may cause suppression of clotting factors II, V, VII, and X, and an increase in prothrombin time.
Adverse Reactions
Clinical Trials Experience
- Patients with moderate to severe COPD or COPD patients who are unresponsive to bronchodilators should be monitored closely for COPD exacerbation and fluid retention.
- The following adverse reactions have been associated with use of anabolic steroids: Hepatic: Cholestatic jaundice with, rarely, hepatic necrosis and death. Hepatocellular neoplasms and peliosis hepatis with long-term therapy. Reversible changes in liver function tests also occur including increased bromsulfophthalein (BSP) retention, changes in alkaline phosphatase and increases in serum bilirubin, aspartate aminotransferase (AST, SGOT) and alanine aminotransferase (ALT, SGPT)
- In males:
- Prepubertal: Phallic enlargement and increased frequency or persistence of erections.
- Postpubertal: Inhibition of testicular function, testicular atrophy and oligospermia, impotence, chronic priapism, epididymitis, and bladder irritability.
- In females:
- Clitoromegaly, menstrual irregularities.
CNS
Habituation, excitation, insomnia, depression, and changes in libido.
Hematologic
Bleeding in patients on concomitant oral anticoagulant therapy.
Breast
Larynx
Deepening of the voice in females.
Hair
Hirsutism and male pattern baldness in females.
Skin
Acne (especially in females and prepubertal males).
Skeletal
Premature closure of epiphyses in children.
Fluid and electrolytes
Edema, retention of serum electrolytes (sodium chloride, potassium, phosphate, calcium).
Metabolic/Endocrine
Decreased glucose tolerance, increased creatinine excretion, increased serum levels of creatinine phosphokinase (CPK). Masculinization of the fetus. Inhibition of gonadotropin secretion.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Oxandrolone in the drug label.
Drug Interactions
- Anticoagulants
- Anabolic steroids may increase sensitivity to oral anticoagulants. Dosage of the anticoagulant may have to be decreased in order to maintain desired prothrombin time. Patients receiving oral anticoagulant therapy require close monitoring, especially when anabolic steroids are started or stopped.
- Warfarin
- A multidose study of oxandrolone, given as 5 or 10 mg bid in 15 healthy subjects concurrently treated with warfarin, resulted in a mean increase in S-warfarin half-life from 26 to 48 hours and AUC from 4.55 to 12.08 ng*hr/mL; similar increases in R-warfarin half-life and AUC were also detected. Microscopic hematuria (9/15) and gingival bleeding (1/15) were also observed. A 5.5-fold decrease in the mean warfarin dose from 6.13 mg/day to 1.13 mg/day (approximately 80-85% reduction of warfarin dose), was necessary to maintain a target INR of 1.5. When oxandrolone therapy is initiated in a patient already receiving treatment with warfarin, the INR or prothrombin time (PT) should be monitored closely and the dose of warfarin adjusted as necessary until a stable target INR or PT has been achieved. Furthermore, in patients receiving both drugs, careful monitoring of the INR or PT, and adjustment of the warfarin dosage if indicated are recommended when the oxandrolone dose is changed or discontinued. Patients should be closely monitored for signs and symptoms of occult bleeding.
- Oral hypoglycemic agents
- Oxandrolone may inhibit the metabolism of oral hypoglycemic agents.
- Adrenal steroids or ACTH
Use in Specific Populations
Pregnancy
- Pregnancy Category X
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Oxandrolone in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Oxandrolone during labor and delivery.
Nursing Mothers
- It is not known whether anabolic steroids are excreted in human milk. Because of the potential of serious adverse reactions in nursing infants from oxandrolone, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Anabolic agents may accelerate epiphyseal maturation more rapidly than linear growth in children and the effect may continue for 6 months after the drug has been stopped. Therefore, therapy should be monitored by x-ray studies at 6-month intervals in order to avoid the risk of compromising adult height. Androgenic anabolic steroid therapy should be used very cautiously in children and only by specialists who are aware of the effects on bone maturation.
Geriatic Use
There is no FDA guidance on the use of Oxandrolone with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Oxandrolone with respect to specific gender populations.
Race
There is no FDA guidance on the use of Oxandrolone with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Oxandrolone in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Oxandrolone in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Oxandrolone in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Oxandrolone in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Oxandrolone in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Oxandrolone in the drug label.
Overdosage
Acute Overdose
- No symptoms or signs associated with overdosage have been reported. It is possible that sodium and water retention may occur.
- The oral LD50 of oxandrolone in mice and dogs is greater than 5,000 mg/kg. No specific antidote is known, but gastric lavage may be used.
Chronic Overdose
There is limited information regarding Chronic Overdose of Oxandrolone in the drug label.
Pharmacology
| |
| |
Oxandrolone
| |
| Systematic (IUPAC) name | |
| 17β-hydroxy-17α-methyl-2-oxa-5α-androstan-3-one | |
| Identifiers | |
| CAS number | |
| ATC code | A14 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 306.44 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 97% |
| Metabolism | Hepatic |
| Half life | 9 hours |
| Excretion | Urinary:90%; Fecal:7% |
| Therapeutic considerations | |
| Pregnancy cat. |
X |
| Legal status |
Schedule III (US) |
| Routes | Oral |
Mechanism of Action
- Anabolic steroids are synthetic derivatives of testosterone. Certain clinical effects and adverse reactions demonstrate the androgenic properties of this class of drugs. Complete dissociation of anabolic and androgenic effects has not been achieved. The actions of anabolic steroids are therefore similar to those of male sex hormones with the possibility of causing serious disturbances of growth and sexual development if given to young children. Anabolic steroids suppress the gonadotropic functions of the pituitary and may exert a direct effect upon the testes.
- During exogenous administration of anabolic androgens, endogenous testosterone release is inhibited through inhibition of pituitary luteinizing hormone (LH). At large doses, spermatogenesis may be suppressed through feedback inhibition of pituitary follicle-stimulating hormone (FSH)
Structure
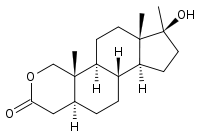
- Oxandrolone oral tablets contain 2.5 mg or 10 mg of the anabolic steroid oxandrolone. Oxandrolone is 17β-hydroxy-17α-methyl-2-oxa-5α-androstan-3-one with the following structural formula:
- Inactive ingredients include cornstarch, lactose, magnesium stearate, and hydroxypropyl methylcellulose.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Oxandrolone in the drug label.
Pharmacokinetics
- In a single dose pharmacokinetic study of oxandrolone in elderly subjects, the mean elimination half-life was 13.3 hours. In a previous single dose pharmacokinetic study in younger volunteers, the mean elimination half-life was 10.4 hours. No significant differences between younger and elderly volunteers were found for time to peak, peak plasma concentration or AUC after a single dose of oxandrolone. The correlation between plasma level and therapeutic effect has not been defined.
Nonclinical Toxicology
- Oxandrolone has not been tested in laboratory animals for carcinogenic or mutagenic effects. In 2-year chronic oral rat studies, a dose-related reduction of spermatogenesis and decreased organ weights (testes, prostate, seminal vesicles, ovaries, uterus, adrenals, and pituitary) were shown.
Clinical Studies
There is limited information regarding Clinical Studies of Oxandrolone in the drug label.
How Supplied
- Oxandrolone 2.5 mg tablets are oval, white, and scored with OX on one side and “11” on each side of the scoreline on the other side; bottles of 100 (NDC 0591-3544-01).
- Oxandrolone 10 mg tablets are capsule shaped, white, with OX on one side and “10” on the other side; bottles of 60 (NDC 0591-3545-60).
Storage
There is limited information regarding Oxandrolone Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Oxandrolone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Oxandrolone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- The physician should instruct patients to report immediately any use of warfarin and any bleeding.
- The physician should instruct patients to report any of the following side effects of androgens:
- Males: Too frequent or persistent erections of the penis, appearance or aggravation of acne.
- Females: Hoarseness, acne, changes in menstrual periods, or more facial hair.
Precautions with Alcohol
- Alcohol-Oxandrolone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- OXANDROLONE®[6]
Look-Alike Drug Names
There is limited information regarding Oxandrolone Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Mendenhall CL, Anderson S, Garcia-Pont P, Goldberg S, Kiernan T, Seeff LB; et al. (1984). "Short-term and long-term survival in patients with alcoholic hepatitis treated with oxandrolone and prednisolone". N Engl J Med. 311 (23): 1464–70. doi:10.1056/NEJM198412063112302. PMID 6390194.
- ↑ Demling RH, DeSanti L (1997). "Oxandrolone, an anabolic steroid, significantly increases the rate of weight gain in the recovery phase after major burns". J Trauma. 43 (1): 47–51. PMID 9253907.
- ↑ Grunfeld C, Kotler DP, Dobs A, Glesby M, Bhasin S (2006). "Oxandrolone in the treatment of HIV-associated weight loss in men: a randomized, double-blind, placebo-controlled study". J Acquir Immune Defic Syndr. 41 (3): 304–14. doi:10.1097/01.qai.0000197546.56131.40. PMID 16540931.
- ↑ Porro LJ, Herndon DN, Rodriguez NA, Jennings K, Klein GL, Mlcak RP; et al. (2012). "Five-year outcomes after oxandrolone administration in severely burned children: a randomized clinical trial of safety and efficacy". J Am Coll Surg. 214 (4): 489–502, discussion 502-4. doi:10.1016/j.jamcollsurg.2011.12.038. PMC 3412530. PMID 22463890.
- ↑ Urban MD, Lee PA, Dorst JP, Plotnick LP, Migeon CJ (1979). "Oxandrolone therapy in patients with Turner syndrome". J Pediatr. 94 (5): 823–7. PMID 448501.
- ↑ "OXANDROLONE oxandrolone tablet".
{{#subobject:
|Page Name=Oxandrolone |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Oxandrolone |Label Name=Oxandrolone02.png
}}
{{#subobject:
|Label Page=Oxandrolone |Label Name=Oxandrolone03.png
}}
{{#subobject:
|Label Page=Oxandrolone |Label Name=Oxandrolone04.png
}}
