Nadolol (tablet)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alonso Alvarado, M.D. [2], Sheng Shi, M.D. [3], Rabin Bista, M.B.B.S. [4]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
Exacerbation of Ischemic Heart Disease Following Abrupt Withdrawal: Hypersensitivity to catecholamines has been observed in patients withdrawn from beta-blocker therapy; exacerbation of angina and, in some cases, myocardial infarction have occurred after abrupt discontinuation of such therapy. When discontinuing chronically administered nadolol, particularly in patients with ischemic heart disease, the dosage should be gradually reduced over a period of one to two weeks and the patient should be carefully monitored. If angina markedly worsens or acute coronary insufficiency develops, nadolol administration should be reinstituted promptly, at least temporarily, and other measures appropriate for the management of unstable angina should be taken. Patients should be warned against interruption or discontinuation of therapy without the physician's advice. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue nadolol therapy abruptly even in patients treated only for hypertension.
|
Overview
Nadolol (tablet) is a beta-adrenergic blocker that is FDA approved for the treatment of agina pectoris, hypertension. There is a Black Box Warning for this drug as shown here. Common adverse reactions include bradyarrhythmia, dizziness, fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Angina Pectoris
- Dosing infromation
- The usual initial dose is 40 mg nadolol once daily. Dosage may be gradually increased in 40 to 80 mg increments at 3 to 7 day intervals until optimum clinical response is obtained or there is pronounced slowing of the heart rate. The usual maintenance dose is 40 or 80 mg administered once daily. Doses up to 160 or 240 mg administered once daily may be needed.
- The usefulness and safety in angina pectoris of dosage exceeding 240 mg per day have not been established. If treatment is to be discontinued, reduce the dosage gradually over a period of one to two weeks
Hypertension
- Dosing infromation
- The usual initial dose is 40 mg nadolol once daily, whether it is used alone or in addition to diuretic therapy. Dosage may be gradually increased in 40 to 80 mg increments until optimum blood pressure reduction is achieved. The usual maintenance dose is 40 or 80 mg administered once daily. Doses up to 240 or 320 mg administered once daily may be needed.
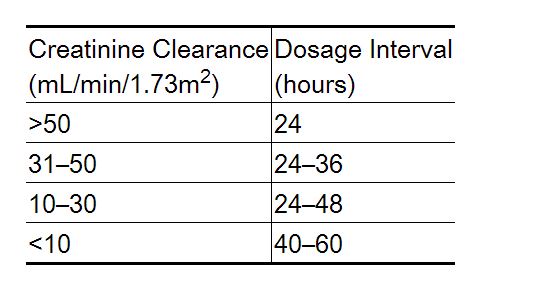
Dosage Adjustment in Renal Failure
- Absorbed nadolol is excreted principally by the kidneys and, although nonrenal elimination does occur, dosage adjustments are necessary in patients with renal impairment. The following dose intervals are recommended:
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Atrial Fibrillation, Heart Rate Control
- Developed by: AHA/ACC
- Class of Recommendation: Class I
- Strength of Evidence: Level B
- Dosing Information/Recommendation
- 10 to 240 mg PO daily[1]
Non–Guideline-Supported Use
Supraventricular Tachycardia
- Dosing information
- Initial IV dose of 0.01 followed by 0.02 and then 0.04 mg/kg administered every 10 minutes until achieving a positive response or the maximum dose of 0.07 mg/kg is reached. Do not exceed 10 mg.[2]
Gastrointestinal Hemorrhage
- Dosing Information
- 40 to 160 mg/day[3]
Hyperthyroidism
- Dosing Information
- 80 to 160 mg/day[4]
Migraine Prophylaxis
- Dosing Information
Tremor
- Dosing information
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Nadolol (tablet) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nadolol in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nadolol in pediatric patients.
Contraindications
- Bronchial asthma
- Sinus bradycardia
- Second degree conduction block
- Third degree conduction block
- Cardiogenic shock
- Cardiac failure
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
Exacerbation of Ischemic Heart Disease Following Abrupt Withdrawal: Hypersensitivity to catecholamines has been observed in patients withdrawn from beta-blocker therapy; exacerbation of angina and, in some cases, myocardial infarction have occurred after abrupt discontinuation of such therapy. When discontinuing chronically administered nadolol, particularly in patients with ischemic heart disease, the dosage should be gradually reduced over a period of one to two weeks and the patient should be carefully monitored. If angina markedly worsens or acute coronary insufficiency develops, nadolol administration should be reinstituted promptly, at least temporarily, and other measures appropriate for the management of unstable angina should be taken. Patients should be warned against interruption or discontinuation of therapy without the physician's advice. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue nadolol therapy abruptly even in patients treated only for hypertension.
|
Cardiac Failure
- Sympathetic stimulation may be a vital component supporting circulatory function in patients with congestive heart failure, and its inhibition by beta-blockade may precipitate more severe failure. Although beta-blockers should be avoided in overt congestive heart failure, if necessary, they can be used with caution in patients with a history of failure who are well-compensated, usually with digitalis and diuretics. Beta-blockers do not abolish the inotropic action of digitalis on heart muscle.
- In patients without a history of heart failure, continued use of beta-blockers can, in some cases, lead to cardiac failure. Therefore, at the first sign or symptom of heart failure, the patient should be digitalized and/or treated with diuretics, and the response observed closely, or nadolol should be discontinued (gradually, if possible).
Nonallergic Bronchospasm (e.g., chronic bronchitis, emphysema)
- Patients with bronchoespastic disease should in general not receive beta-blockers. Nadolol should be administered with caution since it may block bronchodilation produced by endogenous or exogenous catecholamine stimulation of beta2 receptors.
Major Surgery
- Chronically administered beta-blocking therapy should not be routinely withdrawn prior to major surgery; however, the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.
Diabetes and Hypoglycemia
- Beta-blockers may prevent the appearance of premonitory signs and symptoms (e.g., tachycardia and blood pressure changes) of acute hypoglycemia. This is especially important with labile diabetics. Beta-blockade also reduces the release of insulin in response to hyperglycemia; therefore, it may be necessary to adjust the dose of antidiabetic drugs.
Thyrotoxicosis
- Beta-blockers may mask certain clinical signs (e.g., tachycardia) of hyperthyroidism. Patients suspected of developing thyrotoxicosis should be managed carefully to avoid abrupt withdrawal of beta-adrenergic blockade which might precipitate a thyroid storm.
Adverse Reactions
Clinical Trials Experience
Cardiovascular
- Bradycardia with heart rates of less than 60 beats per minute occurs commonly, and heart rates below 40 beats per minute and/or symptomatic bradycardia were seen in about 2 of 100 patients. Symptoms of peripheral vascular insufficiency, usually of the Raynaud type, have occurred in approximately 2 of 100 patients.
- Cardiac failure, hypotension, and rhythm/conduction disturbances have each occurred in about 1 of 100 patients.
- Single instances of first degree heart block and third degree heart block have been reported; intensification of AV block is a known effect of beta-blockers.
Central Nervous System
- Dizziness or fatigue has been reported in approximately 2 of 100 patients.
- Paresthesias, sedation, change in behavior have each been reported in approximately 6 of 1000 patients.
Respiratory
- Bronchospasm has been reported in approximately 1 of 1000 patients.
Gastrointestinal
- Nausea, diarrhea, abdominal discomfort, constipation, vomiting, indigestion, anorexia, bloating, and flatulence have been reported in 1 to 5 of 1000 patients.
Miscellaneous
Each of the following has been reported in 1 to 5 of 1000 patients:
- Rash
- Pruritus
- Headache
- Dry mouth, eyes, or skin
- Impotence or decreased libido
- Facial swelling
- Weight gain
- Slurred speech
- Cough
- Nasal stuffiness
- Sweating
- Tinnitus
- Blurred vision
- Reversible alopecia has been reported infrequently.
The following adverse reactions have been reported in patients taking nadolol and/or other beta-adrenergic blocking agents, but no causal relationship to nadolol has been established.
Central Nervous System
- Reversible mental depression progressing to catatonia
- Visual disturbances
- Hallucinations
- An acute reversible syndrome characterized by disorientation for time and place, short-term memory loss, emotional lability with slightly clouded sensorium, and decreased performance on neuropsychometrics.
Gastrointestinal
Hematologic
Allergic
- Fever combined with aching and sore throat
- Laryngospasm
- Respiratory distress
Miscellaneous
- Pemphigoid rash
- Hypertensive reaction in patients with pheochromocytoma
- Sleep disorders
- Peyronie's disease
- The oculomucocutaneous syndrome associated with the beta-blocker practolol has not been reported with nadolol.
Postmarketing Experience
There is limited information regarding Nadolol (tablet) Postmarketing Experience in the drug label.
Drug Interactions
When administered concurrently, the following drugs may interact with beta-adrenergic receptor blocking agents:
- General anesthetics: Exaggeration of the hypotension induced by general anesthetics.
- Antidiabetic drugs (oral agents and insulin): Hypoglycemia or hyperglycemia; adjust dosage of antidiabetic drug accordingly.
- Catecholamine-depleting drugs (e.g., reserpine): Additive effect; monitor closely for evidence of hypotension and/or excessive bradycardia (e.g., vertigo, syncope, orthostatic hypotension).
- Digitalis glycosides: Both digitalis glycosides and beta-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia.
Response to Treatment for Anaphylactic Reaction: While taking beta-blockers, patients with a history of severe anaphylactic reaction to a variety of allergens may be more reactive to repeated challenge, either accidental, diagnostic, or therapeutic. Such patients may be unresponsive to the usual doses of epinephrine used to treat allergic reaction.
Use in Specific Populations
Pregnancy
- In animal reproduction studies with nadolol, evidence of embryo- and fetotoxicity was found in rabbits, but not in rats or hamsters, at doses 5 to 10 times greater (on a mg/kg basis) than the maximum indicated human dose. No teratogenic potential was observed in any of these species.
- There are no adequate and well-controlled studies in pregnant women. Nadolol should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Neonates whose mothers are receiving nadolol at parturition have exhibited bradycardia, hypoglycemia, and associated symptoms.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Nadolol (tablet) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Nadolol (tablet) during labor and delivery.
Nursing Mothers
- Nadolol is excreted in human milk. Because of the potential for adverse effects in nursing infants, a decision should be made whether to discontinue nursing or to discontinue therapy taking into account the importance of nadolol to the mother.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
There is no FDA guidance on the use of Nadolol (tablet) in geriatric settings.
Gender
There is no FDA guidance on the use of Nadolol (tablet) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Nadolol (tablet) with respect to specific racial populations.
Renal Impairment
Nadolol should be used with caution in patients with impaired renal function.
Hepatic Impairment
There is no FDA guidance on the use of Nadolol (tablet) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Nadolol (tablet) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Nadolol (tablet) in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral/Intravenous
Monitoring
There is limited information about monitoring Nadolol in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Nadolol (tablet) and IV administrations.
Overdosage
- Nadolol can be removed from the general circulation by hemodialysis.
- In addition to gastric lavage, the following measures should be employed, as appropriate. In determining the duration of corrective therapy, note must be taken of the long duration of the effect of nadolol:
- Excessive Bradycardia: Administer atropine (0.25 to 1.0 mg). If there is no response to vagal blockade, administer isoproterenol cautiously.
- Cardiac Failure: Administer a digitalis glycoside and diuretic. It has been reported that glucagon may also be useful in this situation.
- Hypotension: Administer vasopressors, e.g., epinephrine or levarterenol. (There is evidence that epinephrine may be the drug of choice.)
- Bronchospasm: Administer a beta2-stimulating agent and/or a theophylline derivative.
Pharmacology
Mechanism of Action
The mechanism of the antihypertensive effects of beta-adrenergic receptor blocking agents has not been established; however, factors that may be involved include (1) competitive antagonism of catecholamines at peripheral (non-CNS) adrenergic neuron sites (especially cardiac) leading to decreased cardiac output, (2) a central effect leading to reduced tonic-sympathetic nerve outflow to the periphery, and (3) suppression of renin secretion by blockade of the beta-adrenergic receptors responsible for renin release from the kidneys.
- While cardiac output and arterial pressure are reduced by nadolol therapy, renal hemodynamics are stable, with preservation of renal blood flow and glomerular filtration rate.
- By blocking catecholamine-induced increases in heart rate, velocity and extent of myocardial contraction, and blood pressure, nadolol generally reduces the oxygen requirements of the heart at any given level of effort, making it useful for many patients in the long-term management of angina pectoris. On the other hand, nadolol can increase oxygen requirements by increasing left ventricular fiber length and end diastolic pressure, particularly in patients with heart failure.
- Although beta-adrenergic receptor blockade is useful in treatment of angina and hypertension, there are also situations in which sympathetic stimulation is vital. For example, in patients with severely damaged hearts, adequate ventricular function may depend on sympathetic drive. Beta-adrenergic blockade may worsen AV block by preventing the necessary facilitating effects of sympathetic activity on conduction. Beta2-adrenergic blockade results in passive bronchial constriction by interfering with endogenous adrenergic bronchodilator activity in patients subject to bronchospasm and may also interfere with exogenous bronchodilators in such patients.
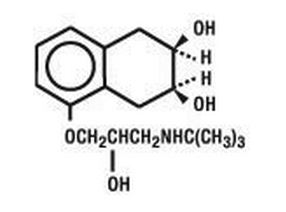
Structure
- Nadolol is a synthetic nonselective beta-adrenergic receptor blocking agent designated chemically as 1-(tert-butylamino)-3-[(5,6,7,8-tetrahydro-cis-6,7-dihydroxy-1-naphthyl)oxy]-2-propanol. Structural formula:
- Nadolol is a white crystalline powder. It is freely soluble in ethanol, soluble in hydrochloric acid, slightly soluble in water and in chloroform, and very slightly soluble in sodium hydroxide.
- Nadolol is available for oral administration as 20 mg, 40 mg, and 80 mg tablets. Inactive ingredients: microcrystalline cellulose, colorant (FD&C Blue No. 2), corn starch, magnesium stearate, povidone (except 20 mg and 40 mg), and other ingredients.
Pharmacodynamics
- Clinical pharmacology studies have demonstrated beta-blocking activity by showing (1) reduction in heart rate and cardiac output at rest and on exercise, (2) reduction of systolic blood pressure and diastolic blood pressure at rest and on exercise, (3) inhibition of isoproterenol-induced tachycardia, and (4) reduction of reflex orthostatic tachycardia.
- Nadolol specifically competes with beta-adrenergic receptor agonists for available beta receptor sites; it inhibits both the beta1 receptors located chiefly in cardiac muscle and the beta2 receptors located chiefly in the bronchial and vascular musculature, inhibiting the chronotropic, inotropic, and vasodilator responses to beta-adrenergic stimulation proportionately. Nadolol has no intrinsic sympathomimetic activity and, unlike some other beta-blockers, nadolol has little direct myocardial depressant activity and does not have an anesthetic-like membrane- stabilizing action. Animal and human studies show that nadolol slows the sinus rate and depresses AV conduction. In dogs, only minimal amounts of nadolol were detected in the brain relative to amounts in blood and other organs and tissues. Nadolol has low lipophilicity as determined by octanol/water partition coefficient, a characteristic of certain beta-blockers that has been correlated with the limited extent to which these agents cross the blood-brain barrier, their low concentration in the brain, and low incidence of CNS-related side effects.
- In controlled clinical studies, nadolol at doses of 40 to 320 mg/day has been shown to decrease both standing and supine blood pressure, the effect persisting for approximately 24 hours after dosing.
Pharmacokinetics
- Absorption: Absorption of nadolol after oral dosing is variable, averaging about 30 percent. Peak serum concentrations of nadolol usually occur in three to four hours after oral administration and the presence of food in the gastrointestinal tract does not affect the rate or extent of nadolol absorption.
- Distribution: Approximately 30 percent of the nadolol present in serum is reversibly bound to plasma protein.
- Metabolism: Unlike many other beta-adrenergic blocking agents, nadolol is not metabolized by the liver and is excreted unchanged, principally by the kidneys.
- Elimination: The half-life of therapeutic doses of nadolol is about 20 to 24 hours, permitting once-daily dosage. Because nadolol is excreted predominantly in the urine, its half-life increases in renal failure. Steady-state serum concentrations of nadolol are attained in six to nine days with once-daily dosage in persons with normal renal function. Because of variable absorption and different individual responsiveness, the proper dosage must be determined by titration.
Nonclinical Toxicology
- Carcinogenesis
- Mutagenesis
- Impairment of Fertility
- In chronic oral toxicologic studies (one to two years) in mice, rats, and dogs, nadolol did not produce any significant toxic effects. In two-year oral carcinogenic studies in rats and mice, nadolol did not produce any neoplastic, preneoplastic, or non-neoplastic pathologic lesions. In fertility and general reproductive performance studies in rats, nadolol caused no adverse effects.
Clinical Studies
There is limited information regarding Nadolol (tablet) Clinical Studies in the drug label.
How Supplied
- Nadolol tablets USP for oral administration are available as:
- 20 mg:White, round, uncoated tablets stamped SZ above and 465 below the bisect line on one side and plain on the other side and supplied as (NDC 68001-221-00 bottles of 100)
- 40 mg: White, round, uncoated tablets stamped SZ above and 466 below the bisect line on one side and plain on the other side and supplied as (NDC 68001-220-00 bottles of 100), (NDC 68001-220-08 bottles of 1000).
- 80 mg: White, round, uncoated tablets stamped SZ above and 467 below the bisect line on one side and plain on the other side and supplied as (NDC 68001-219-00 bottles of 100)
Storage
- Store at 20°-25°C (68°-77°F).
- Protect from moisture, freezing and excessive heat.
- Dispense in a tight container as defined in the USP.
Images
Drug Images
{{#ask: Page Name::Nadolol (tablet) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Nadolol (tablet) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients, especially those with evidence of coronary artery insufficiency, should be warned against interruption or discontinuation of nadolol therapy without the physician's advice. Although cardiac failure rarely occurs in properly selected patients, patients being treated with beta-blockers should be advised to consult the physician at the first sign or symptom of impending failure. The patient should also be advised of a proper course in the event of an inadvertently missed dose.
Precautions with Alcohol
Alcohol-Nadolol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Corgard
Look-Alike Drug Names
There is limited information regarding Nadolol (tablet) Look-Alike Drug Names in the drug label.
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Cigarroa JE; et al. (2014). "2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society". J Am Coll Cardiol. doi:10.1016/j.jacc.2014.03.022. PMID 24685669.
- ↑ Olukotun AY, Klein GJ (1987). "Efficacy and safety of intravenous nadolol for supraventricular tachycardia". Am J Cardiol. 60 (6): 59D–62D. PMID 3630923.
- ↑ Gatta A, Merkel C, Sacerdoti D, Bolognesi M, Caregaro L, Zuin R; et al. (1987). "Nadolol for prevention of variceal rebleeding in cirrhosis: a controlled clinical trial". Digestion. 37 (1): 22–8. PMID 3301478.
- ↑ Lazarus JH, Kingswood JC, John R (1987). "The effect of nadolol on heart rate in hyperthyroidism. A controlled trial". Acta Endocrinol (Copenh). 114 (1): 102–6. PMID 3544631.
- ↑ Pascual J, Rivas MT, Leira R (2007). "Testing the combination beta-blocker plus topiramate in refractory migraine". Acta Neurol Scand. 115 (2): 81–3. doi:10.1111/j.1600-0404.2006.00772.x. PMID 17212609.
- ↑ Ryan RE, Ryan RE, Sudilovsky A (1983). "Nadolol: its use in the prophylactic treatment of migraine". Headache. 23 (1): 26–31. PMID 6131052.
- ↑ Edwards RV (1982). "Nadolol use for cerebellar tremor". Am J Psychiatry. 139 (11): 1522. PMID 6127958.
- ↑ Koller WC (1983). "Nadolol in essential tremor". Neurology. 33 (8): 1076–7. PMID 6348587.
{{#subobject:
|Label Page=Nadolol (tablet) |Label Name=NadololPackage20mg.png
}}
{{#subobject:
|Label Page=Nadolol (tablet) |Label Name=NadololPackage40mg.png
}}
{{#subobject:
|Label Page=Nadolol (tablet) |Label Name=NadololPackage80mg.png
}}